PLX-200 for Krabbe Disease Earns Orphan Drug Designation
In the United States, Orphan Drug designation is granted to drugs or biologics intended to treat rare or chronic illnesses. For the purpose of this designation, "rare illnesses" are defined…
Jessica Lynn has an educational background in writing and marketing. She firmly believes in the power of writing in amplifying voices, and looks forward to doing so for the rare disease community.
In the United States, Orphan Drug designation is granted to drugs or biologics intended to treat rare or chronic illnesses. For the purpose of this designation, "rare illnesses" are defined…
Genetics play a huge role in health and disease development. Certain genetic mutations are associated with the development of specific conditions; for example, DMD gene mutations cause Duchenne muscular dystrophy.…
According to Pharmaceutical Business Review, Abecma (idecabtagene vicleucel), a CAR T-cell therapy designed to treat patients with relapsed and refractory (R/R) multiple myeloma (MM), received conditional marketing authorization from the…
Clinical trials are often used to understand the safety, efficacy, and tolerability of specific treatments. Unfortunately, sometimes the drugs in these studies fail. According to Targeted Oncology, this occurred in…
In the past, researchers have noted an association between neuroinflammation (inflammation of nervous system tissue) and Alzheimer’s disease. However, researchers often believed that neuroinflammation was more of a result of…
In the past, atrial fibrillation (AFib), the most commonly seen heart arrhythmia, was linked to chronic alcohol abuse or long-term alcohol consumption. However, a new study has shown that drinking…
If a community has more cases of a certain illness, would that make the illness worse or more symptomatic? According to Medical XPress, researchers explored this question in relation to…
Even though some patients have the same condition, the treatment plan differs. Why is that? Cure Today spoke to Dr. Elizabeth Bengtson, MD, a hematology expert, to learn more…

At Patient Worthy, we love to see patient advocates and the impact that these advocates have on their communities. For example, brothers Ethan and Gavin Morrobel have worked tirelessly as…
Clinical trials can be beneficial for finding and evaluating new treatment options. Recently, biopharmaceutical company AstraZeneca shared how ALXN1840, a potential treatment for patients with Wilson disease, reached its primary…
The National Organization for Rare Disorders (NORD) has been a leading patient advocacy organization since the 1980s, helping advance the identification, treatment, and cure of rare disorders. In October 2021,…
Ashley Monroe is an extremely talented singer-songwriter who hails from Tennessee. Over the years, Ashley has grown her music career alone and as part of the Pistol Annies. But now…
When it comes to pharmaceuticals, licensed territory helps ensure that drugs within a certain territory are offered to patients. Exclusive licenses sometimes mean that pharmaceuticals are only available in specific…
For rare diseases in which no current treatment options are available, such as GM1 gangliosidosis, there is an urgent need to find therapeutics and improve patient outcomes. Gene therapy company…
Each year, the National Kidney Foundation (NKF) holds a Kidney Walk to help provide support and assistance to those with chronic kidney disease (CKD) and other kidney-related conditions. Due to…
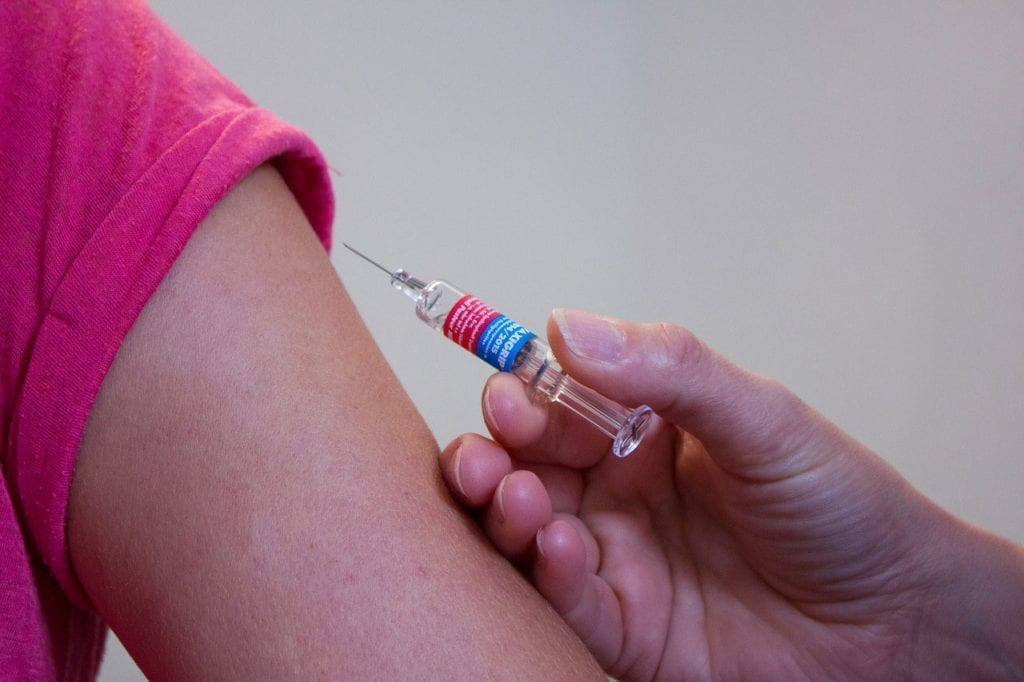
Have you ever wanted to be part of medical research? Well, now you might have the chance. According to Fox2Now, researchers from Saint Louis University - and, in particular, the…
Nearly all cases of Wilms tumors, a rare form of pediatric kidney cancer, are diagnosed before a child turns 10 years old. When Maddie Newburn was first diagnosed, she was…
In the United States, Orphan Drug designation is granted to drugs or biologics intended to treat patients with rare conditions, defined as affecting fewer than 200,000 Americans. As incentives, drug…
When Karter was first born 14 months ago, his mother Breonna was overjoyed. But she quickly realized a series of upcoming obstacles when, at birth, Karter was diagnosed with Alagille…
Normally, the standard FDA review process for potential drugs or biologics takes around 10 months to complete. However, when a drug receives Priority Review status, it means that the FDA…
According to a recent press release from Pfizer and biotechnology company Vivet Therapeutics, VTX-801, a gene therapy candidate for patients with Wilson disease, recently received Fast Track designation from the…

When organizations partner together to work towards improving patient outcomes, it can also help to increase the understanding and spur research of certain conditions. According to Healio, a recent…

Normally, Fast Track is an FDA process designed to facilitate and expedite the development and review of drugs or biologics intended to treat rare or serious conditions. Recently, Healio…

On August 17, 2021, Amnesty International released an urgent call to action to provide protection in Malawi for those with albinism. This call for change came following the death…
Sometimes, it can be difficult to acquire treatment options for patients with rare diseases. Brand name products may also be expensive and relatively inaccessible, prompting the need for generic…