According to a story from ANCA Vasculitis News, the drug developer InflaRx has recently announced that the first patient has been dosed with IFX-1, an experimental therapy which is being tested as a treatment for ANCA vasculitis, a rare autoimmune disease. The dosing signals the initiation of the company’s phase 2 clinical trial. IFX-1 is InflaRx’s leading investigational drug candidate.
About ANCA Vasculitis
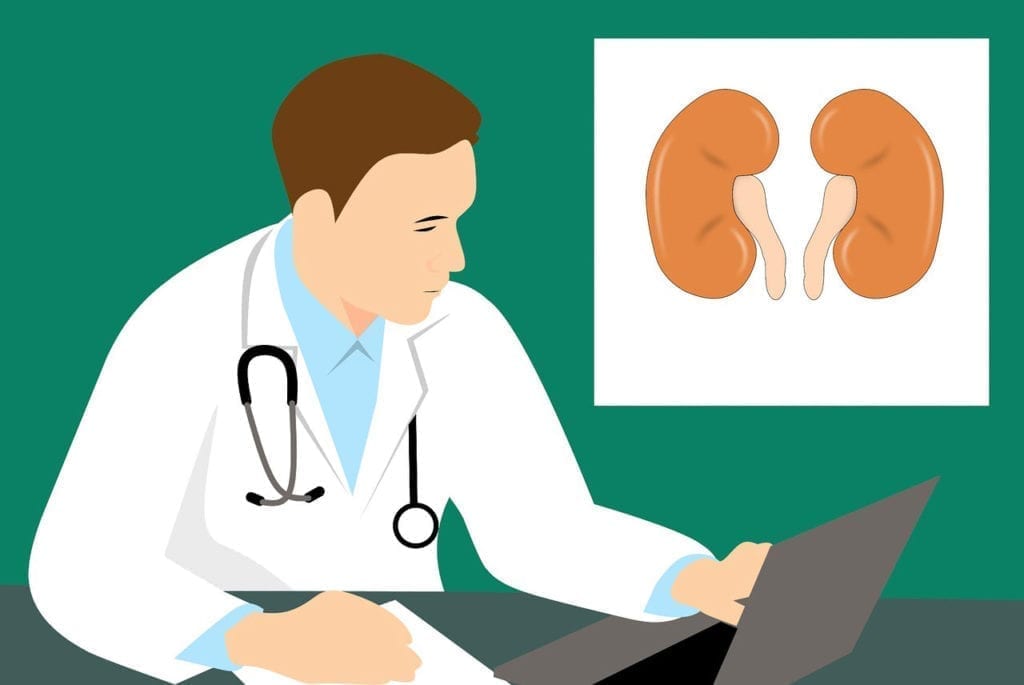
ANCA vasculitis is a disease that is characterized by the damage and destruction of blood vessels as a result of inflammatory activity. The disease is associated with the presence of anti-neutrophil cytoplasmic antibodies (ANCA). These are autoantibodies that target antigens present on neutrophils (the most common type of white blood cell) and monocytes. This means that the mechanism of the disease is autoimmune, in which the body’s own immune system mistakenly attacks healthy body tissue. Symptoms of ANCA vasculitis include kidney inflammation, fever, weight loss, abdominal pain, bloody stools, purpura, nose bleeds, muscle pain, arthritis, bloody cough, vision problems, headaches, stroke, heart attack, and high blood pressure. Treatment of the disease is primarily focused on controlling inflammation and suppressing immune system activity. Common medications include cyclophosphamide, rituximab, and prednisone. Antibiotics may be necessary in cases of infection. To learn more about ANCA vasculitis, click here.
About IFX-1
The launch of this trial was first announced in July of last year. IFX-1 is an antibody that is tailored to inhibit the activation of a certain protein called C5a. This protein is a component of the body’s complement system (which plays an essential role in immune system activity) and prior research suggests that it could contribute to overactive immune system that occurs in ANCA vasculitis.
About The Clinical Trial
The clinical trial will compare IFX-1 alongside a placebo group in ANCA vasculitis patients that are currently using standard immunosuppressive therapies. The trial is expected to involve 80 participants in total and will include patients from 13 different countries. There are a total of 60 sites where the trial will be conducted.
The first portion will see all patients receive treatment with glucocorticoids and either placebo or IFX-1. If the results are positive, the trial will proceed to the second portion, in which the drug will be compared both on its own and as a combination treatment with glucocorticoids.