Research findings can sometimes come from unexpected places. For example, according to Parkinson’s News Today, the benefits and shortcomings of an investigative gene therapy were explored through the post-mortem analysis of two patients with Parkinson’s disease.
The therapy, CERE-120, delivers the gene neurturin (NRTN) into the brain. Admittedly, this had few therapeutic benefits during clinical trials. However, researchers believe that the long-term post-mortem brain analysis highlights where the therapy could be improved to enhance future efficacy. Read the full study in Brain here.
What is Parkinson’s disease?
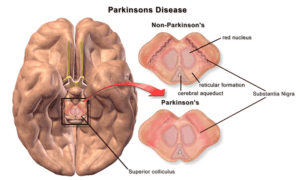
Parkinson’s disease is a progressive central nervous system disorder that occurs in five stages:
- Mild symptoms and light tremors
- More noticeable symptoms with light tremors and muscle rigidity
- Loss of balance and slow movement
- Strong symptoms that hinder independent living
- Patients lose ability to stand and walk, may experience hallucinations
Earlier symptoms include tremors or shaking, specifically in the hands, alongside stiff muscles, impaired balance, and speech changes. Parkinson’s disease occurs when nerve cells in the brain degenerate, die, or are damaged in some way. These nerve cells create dopamine, which normally helps transmit messages to the muscles. Without having this valuable dopamine, motor function is reduced. Learn more about Parkinson’s disease on our website.
Gene Therapy
CERE-120
CERE-120 was first developed by biotechnology company Ceregene. This investigative gene therapy aimed to protect the brain neurons affected by Parkinson’s disease. CERE-120 works by delivering neurturin (NRTN) directly to two areas of the brain: the putamen and the substantia nigra. These are known for their role in movement. NRTN specifically combats neurodegeneration.
This therapy had contrasting results in animal and human trials. In animal models of Parkinson’s disease, an adeno-associated virus serotype 2 implanted NRTN in the brain. Researchers found that neurons associated with dopamine were protected, increasing dopamine production.
Following the results from the animal trials, researchers moved into an open-label Phase 1 clinical trial. This was initially promising. Patients with Parkinson’s disease tolerated the therapy well, suggesting that it was safe for use. This trial also found some improvement in symptoms. At the same time, a double-blind Phase 2 trial showed that CERE-120 had no real impact on patients in comparison to those not given CERE-120.
A following study sought to deliver a higher dose of CERE-120 to the putamen and to the surrounding substantia nigra. Once again, patients showed no symptom improvement.
Post-Mortem Patient Studies
Researchers wanted to understand why the successful results from the Phase 1 clinical trial were not replicated in further studies. So they conducted additional post-mortem (following death) analyses of two patients with Parkinson’s disease who had participated in the clinical trials for CERE-120.
The Patients
The first patient was involved in the first Phase 2 trial for CERE-120, which implanted the therapy only in the putamen. Patient 1 survived for ten years following the surgery.
The second patient was involved in the second Phase 2 trial for CERE-120, which implanted the therapy in both the putamen and substantia nigra. Patient 2 survived for eight years following the surgery.
To ensure the accuracy of their studies, the researchers also examined the brains of four more individuals. Two were patients with Parkinson’s disease around the same age as Patients 1 and 2. These had not been treated with gene therapy. The final two brains studied were from two individuals of a similar age who had no neurological illnesses, including Parkinson’s disease.
Findings
Patient 1 and 2 both exhibited NRTN production in the putamen. Their brains also had increased tyrosine hydroxylase (TH) enzymes; this is key because this enzyme actually helps produce dopamine. While both patients exhibited TH enzymes, they were higher in Patient 2. This suggests that the therapy could be more effective if delivered to multiple areas of the brain, as opposed to one singular delivery.
Researchers found NRTN in 19% of remaining dopaminergic (relating to or producing dopamine) neurons in Patient 1’s brain. Patient 2’s brain showed NRTN in 39% of remaining neurons. The remaining four brains showed no NRTN.
A receptor called RET was also found more highly concentrated in the brain of Patient 2 than Patient 1. Some prior research suggests that protein buildup of alpha-synuclein proteins, which occurs in Parkinson’s disease, can interrupt RET and stop the production of NRTN.
Ultimately, this research opens the door to new considerations. In the moment, CERE-120 gene therapy may not provide enough NRTN to treat Parkinson’s disease. However, the post-mortem studies suggest that gene therapy can contribute to the long-term production of TH in patients. If researchers can find ways to increase that production and see results faster, gene therapy may become an effective treatment for patients with Parkinson’s disease.