Without treatment, an estimated 20% of those with progressive keratoconus may eventually require corneal transplantation. Glaukos, a company creating novel therapies for chronic eye diseases, developed the iLink corneal cross-linking procedure to help preserve vision and slow keratoconus progression. On March 14, 2022, Glaukos announced via news release that it had launched a Phase 2 clinical program, consisting of two clinical trials, to evaluate the 3rd-generation iLink treatment.
About Keratoconus
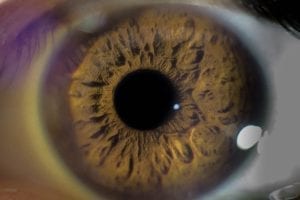
Keratoconus is a relatively rare vision disorder characterized by a thinning cornea. Normally, the cornea, or the clear outer lens of the eye, is dome-shaped. But in keratoconus, the thinning of the cornea causes it to bulge into a conical shape. While doctors are not sure what causes keratoconus, many believe that genetic and environmental factors could play a role. This condition is also more common in individuals with Leber congenital amaurosis, Down syndrome, and Marfan syndrome. Additional risk factors include a family history of keratoconus, frequent eye-rubbing (possibly due to inflammation or itching), being Black or Latino, or being younger in age. Keratoconus typically manifests between the ages of 10-30, and may progress for an additional 10-20 years. While this condition typically affects both eyes, it may progress differently in each eye. Symptoms include:
- Blurred vision
- Increased sensitivity to light
- Worsening or clouded vision with a sudden onset
- Eye irritation
- Headaches due to eye pain
- A need for frequent changes in eyeglass prescriptions
- Difficulty seeing at night
- Halos around bright lights
Learn more about keratoconus.
iLink Corneal Cross-Linking
According to Glaukos, the iLink procedure is the first FDA-approved procedure of its kind. Glaukos has been developing this therapy to optimize patient outcomes. Its first iLink treatment was the iLink Epi-off, with the second being the iLink Epi-on. Now, the corneal cross-linking procedure represents the third generation of Glaukos’ technology.
The procedure consists of the KXL System, which provides UV light and bio-activates the Photrexa or Photrexa Viscous ophthalmic solutions. During the procedure, the UV light helps to stabilize the cornea.
Within these upcoming Phase 2 clinical trials, researchers will analyze the safety, efficacy, and tolerability of the newest generation of iLink. The trial will enroll patients on a global scale. During the trial, researchers will also determine the efficacy of custom vs. non-custom therapeutic patterns.
To learn more about the prior studies performed on iLink, take a look at this clinical data.