Novartis AG, with corporate headquarters based in Basel, Switzerland, recently announced positive results from its Phase III trials REMIX-1 (NCT05030311) and REMIX-2 (NCT05032157) ).
The news was delivered via Drugs.com. During the trials, patients were treated with Remibrutinib, an oral Bruton’s tyrosine kinase (BTK) inhibitor, against chronic spontaneous uticaria (CSU) treatment. Remibrutinib is an oral BTK inhibitor with a positive safety profile.
Remibrutinib blocks the BTK signaling cascade. Thus it prevents histamine release. Histamine causes hives, swelling, and itch.
Patients enrolled in the two Remix trials currently will receive treatment for fifty-two weeks. They will also be able to continue their treatment in a longer extension trial.
About CSU
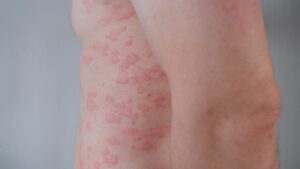
The medical term for hives lasting six weeks or more is CSU with an internal underlying cause rather than an external exposure.
The disorder is characterized by welts or tissue swelling under the skin on the hands, feet, throat, and face and at times accompanied by stinging and burning. The symptoms may last six weeks or longer.
Well under 1% of people in the United States have USC. To date, H1-antihistamines have been the preferred medication for USC. Some studies have found that over 50% of USC patients are unable to find adequate disease control.
Remibrutinib is also being tested to treat immune-mediated disorders including multiple sclerosis, Sjögren’s syndrome, hidradenitis suppurativa, and food allergies.
Fast Acting Results
Patients taking Remibrutinib saw improvement within two weeks after they began treatment.
If Remibrutinib is approved, based upon the favorable reports from the two Phase III trials, it would be the first approved drug in a new class of chronic USC treatment in the past ten years for a disorder that affects over 60 million people in the U.S.
A Third Option
Injectable biologic treatment has been effective where antihistamines failed yet only about twenty percent of patients in the world have been treated with injectables.
Although injectable biologic (natural or used in nature) therapies have proved to be effective, only about twenty percent of patients in the world have received the treatments.
The study has a fifty-two-week end date, at which time data supporting Remibrutinib as an effective, fast-acting treatment option will be presented. The data will be submitted to global health authorities in 2024.
If Remibrutinib is approved based on the favorable reports from the two Phase III trials, it would be the first approved drug in a new class of chronic USC treatment in the past ten years for a disorder that affects over 60 million people in the U.S.