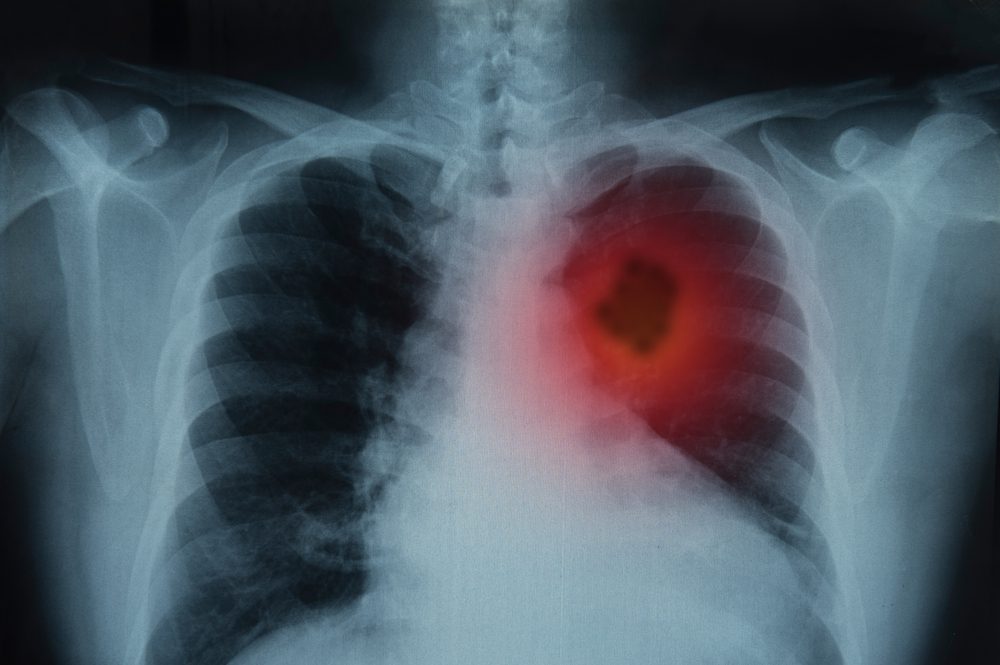
The 2023 World Conference on Lung Cancer took place from September 9-12 this year. During the conference, stakeholders came together to discuss novel research, emerging trends, and other needs and advancements within the lung cancer space. One presentation, as reported by Cancer Network, centered around the results from the dose expansion and escalation portions of the Phase 1/2 KRYSTAL-1 clinical study. This study evaluated adagrasib (marketed under the brand name Krazati) for people living with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC).
Altogether, 132 adult participants enrolled in the study. The majority of patients were older in age, female, and Caucasian. Additionally, most participants had received platinum and anti-PD-1/PD-L1 therapy.
Unpacking the Research Findings
During the trial, participants received 600mg of adagrasib twice each day. Researchers then evaluated clinical activity, overall response rate, overall survival rate, progression-free survival, and one-year survival rate.
Findings from the study show that:
- Adagrasib treatment was safe and well-tolerated, even if taken for a longer period of time (1 year or more). The most common adverse reaction was diarrhea.
- The median overall survival rate was 14.1 months. 52.8% of participants had a one-year overall survival rate, with 31.3% reaching two years.
- While the median progression-free survival rate was 6.9 months, 35% of participants had progression-free survival for one year, with 13.9% at two years.
- Altogether, 43% of participants had some objective response to treatment.
When considering the subgroups involved in the study, adagrasib was most effective in participants with TP53 and CDKN2A mutations in both median overall survival and progression-free survival. While median overall survival was 9.2 months and 5.7 months in those with STK11 or KEAP1 mutations, progression-free survival was significantly shorter in these groups than in those with TP53 or CDKN2A mutations.
In those whose cancer had metastasized (spread) to their central nervous system, overall survival and progression-free survival sat at 14.7 and 6.9 months, respectively.
Moving forward, researchers plan to continue evaluating adagrasib for previously treated individuals with KRAS G12C-mutated NSCLC in a Phase 3 clinical study. In this study, researchers hope to understand how effective adagrasib is compared to another therapy called docetaxel.
About Non-Small Cell Lung Cancer (NSCLC)
An estimated 80-85% of lung cancer cases are non-small cell lung cancer, though this category can be broken down further based on specific genetic mutations or by the specific cancer subtype (adenocarcinoma, squamous cell carcinoma, large cell carcinoma). While NSCLC is not as aggressive as its counterpart small cell lung cancer, many cases of NSCLC remain undiagnosed until later stages. At the time of diagnosis, the cancer has already metastasized in approximately 40% of those affected.
Doctors have identified risk factors associated with NSCLC development. Smoking is the largest risk factor. Additional risk factors include second-hand smoke exposure, workplace carcinogen or radiation exposure, environmental pollution, HIV, or a family history of lung cancer.
Symptoms of NSCLC may include a progressive cough that may produce blood, wheezing, chest pain or pressure, difficulty breathing, appetite loss and/or unintended weight loss, fatigue, dysphagia (difficulty swallowing), hoarseness, or swelling in the face or neck.