Mahesh Karande, the President and CEO of Omega Therapeutics, is incredibly passionate about epigenetics. And it shows: in the work that he and his team do, in the therapies they are developing, and even in the way that he talks. While explaining the development of OTX-2002 for hepatocellular carcinoma (HCC), Mahesh animatedly shares:
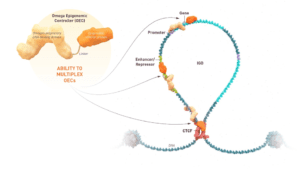
The epigenome is the level above or around the genome. Nature modifies epigenetic marks on regulatory sites which control genes, which are expressed at a certain level. When nature’s programs go awry, diseases happen. We’re using mRNA therapeutics to home into the genome and, exactly like nature would do, delete, modify, or adjust those epigenetic marks to treat or cure that gene.
Recently, this passion and drive has led to OTX-2002 entering the clinical testing realm. The first patient was dosed in a Phase 1/2 trial at the end of October. Since then, OTX-2002 has also received Orphan Drug designation from the U.S. FDA for the treatment of HCC.
In our interview, Mahesh shared his background and why he came onboard at Omega Therapeutics, the future of epigenetic medicine, what OTX-2002 is, and how this therapy has the potential to make huge changes in the realm of HCC.
About Mahesh Karande
Mahesh Karande is the President and CEO of Omega Therapeutics, a clinical-stage biotechnology company that is pioneering the first systematic approach to use mRNA therapeutics as a new class of programmable epigenetic medicines.
Mahesh completed his undergraduate studies in engineering at the University of Bombay before pursuing an M.S. in Engineering from the Georgia Institute of Technology. He explains:
I’m an engineer by training. Once I came to the U.S., I received my M.B.A. from the Wharton School at the University of Pennsylvania. In 2001, I began working as a consultant for McKinsey & Company, which introduced me to the world of pharma and biotech.
Prior to joining Omega just around three and a half years ago, Mahesh worked in various leadership roles at Novartis. He shares:
I worked in emerging markets, so my family and I have gone to Switzerland, Egypt, and South Africa. In Egypt, I was able to launch some specialty drugs like Lucentys for age-related macular degeneration and Gilenya for multiple sclerosis that were at prices affordable to the Egyptian people. In Africa, I was running the general medicine and oncology program. It was amazing to be able to create more access for patients, offering sixteen drugs at $1 per patient per month. Patient-centricity is a huge part of what I do and finding ways to create better access for patients has been a mainstay of my career since the beginning.
According to the Omega Therapeutics “About Us” page, Mahesh also worked as President and CEO of Macrolide Pharmaceuticals prior to joining the team at Omega. Throughout all of his prior and current work experiences, he has played a role in over 10 product launches in various markets.
Joining Omega
So what eventually brought Mahesh over to the Omega Therapeutics team? He explains that this transition began when, after speaking to the company founders, he learned about the science being used to develop various therapies. He shares:
When I heard about their science, I thought that, if it could be translated into a drug development platform, it could transform medicine and what medicine can do. In life, you really only get the chance once, maybe twice if you’re lucky, to join something so transformative. When I’m retired, sitting on my back porch, I want to think about how the work we’ve done has made a difference. And I realized that was what Omega was bringing. We’ve found a way to harness nature’s operating system and control genes, to tune them back to normal range like a thermostat. That’s one of the most powerful things I can think of and it’s so amazing to be able to be part of the work to bring that to fruition.
Mahesh came onboard as the first CEO for Omega. Very soon after, he set up the company ethos: ambitious yet humble. He explains:
We’re going where nobody has gone before. You have to have ambition when you’re doing this. But balancing that, you have to have a tremendous sense of humility. You cannot do this alone; you are standing on the shoulders of giants, the people who came before us. But you’re doing this for patients and you need to earn the right to treat patients with these medicines.
He describes the team at Omega as incredible, hard-working, and dedicated. Moving forward, he is confident that the team can build and engineer medicines for specific diseases and truly make a change in patient lives.

Developing OTX-2002
On October 27, 2022, Omega Therapeutics announced that the first patient was dosed in the Phase 1/2 MYCHELANGELO I clinical study evaluating OTX-2002 for advanced HCC. When asked about the study’s name, Mahesh explained:
Our genes are ubiquitous in every cell of our body and are expressed in a certain way. But when disease occurs, gene expression may change. MYC is the holy grail of genes and presents in a lot of solid tumors and cancers. An estimated 70% of people living with HCC have MYC driving their cancer and an estimated 50% of people living with cancer overall. So the study name – MYChelangelo – is a reference to that gene. Michelangelo was also one of the most famous Renaissance painters. What we’re doing as a pioneering company in this field is creating a renaissance for patients.
An estimated 190 patients will enroll throughout the U.S., Asia, and Europe. Within the trial, researchers will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of OTX-2002. The first portion of the trial (escalation and expansion) will focus on OTX-2002 as a monotherapy. The second portion (safety run-in and expansion) will explore OTX-2002 in combination with current standards-of-care, including TKIs and checkpoint inhibitors. Both portions of the trial will also look at preliminary anti-tumor activity.
Mahesh explains:
The dose escalation phase aims to get us to the right level of the dose for future research. Since this is a first-in-class drug being studied for the first time, we want to take our time. We take patient safety very seriously.
Hepatocellular Carcinoma (HCC): An Overview
Although HCC is rare, it is also considered the most common form of primary liver cancer. As shared, the MYC gene has been implicated in the development of hepatocellular carcinoma. Additional risk factors include hepatitis B or C, obesity, diabetes, pre-existing liver conditions, and heavy alcohol consumption. In many cases, people are asymptomatic in early stages. As the cancer progresses, symptoms can include unintended weight loss, appetite loss, general weakness or fatigue, nausea and vomiting, abdominal bloating, upper right abdominal pain or heaviness, dark urine, and pale bowel movements.
What is OTX-2002?
Mahesh explains OTX-2002 as:
The first-ever Omega Epigenomic Controller that has entered the clinic. It is a programmable mRNA medicine. mRNA has made great strides in terms of vaccinations, but we’re using this for the first time to epigenetically tune c-Myc (MYC) and downregulate its expression. We go to the genome in the liver cells where the gene is dysregulated and overexpressed, and we use epigenetics, which is essentially the most fundamental mechanism used by nature, to tune this gene back to normal. Our hope is that this will resolve the cancer.
In animal models of primary liver tumors, Omega Therapeutics has seen promise from OTX-2002 treatment. From a mechanistic standpoint, the epigenetic changes are able to control gene expression and return it to normal range. Treating the animal models saw tumor cell death via a mechanism called apoptosis and tumor shrinkage. OTX-2002 was also found to be specific to its intended target. Other treatments do not have specificity; they bombard the body, which may lead to unintended side effects. Through preclinical research, Omega Therapeutics has found that OTX-2002 can target and can selectively target tumor cells while protecting normal cells.
Given that there are limited therapeutic options for people with advanced HCC, OTX-2002 has the potential – if effective – to fill an unmet need. Currently, advanced HCC, even when treated, comes with a median overall survival rate of 10-20 months. Nearly 90% of all liver cancer is diagnosed as HCC. Therefore, finding effective therapeutic options is needed.
Soon after the first patient was dosed in the MYCHELANGELO I trial, OTX-2002 received Orphan Drug designation from the FDA. Mahesh shares:
The FDA has recognized the importance of this drug. We’re innovating for patients and the regulatory authorities agree. It is incredible to receive this designation and validation that we’re working in the best interest of patients.
Looking ahead, Omega recently announced a second program targeting MYC in non-small cell lung cancer (NSCLC). The company’s broad pipeline also addresses conditions such as overactive inflammation, liver regeneration, and alopecia. Learn more about Omega Therapeutics’ science and therapeutic development platform.


