Throughout the world, it can be difficult to advance research and drug development in the rare disease space. In part, this is because many rare diseases have small population sizes. It can be hard to find enough people to contribute to research. Developing drugs for these small communities may also not be profitable for pharmaceutical companies, which can have extremely negative impacts on people living with these conditions. The Orphan Drug designation is meant to overcome these obstacles by incentivizing drug development within this space.
In a late June 2023 news release, clinical-stage immuno-oncology company ALX Oncology Holdings Inc. (“ALX Oncology”) shared that the European Commission (“EC”) granted Orphan Drug designation to the company’s lead product candidate evorpacept for gastric cancer and gastroesophageal junction adenocarcinoma. ALX Oncology describes evorpacept as:
a next generation CD47 blocking therapeutic that combines a high-affinity CD47 binding domain with an inactivated, proprietary Fc domain. We believe our inactive Fc approach improves tolerability when compared to other CD47 blocking approaches.
Currently, ALX Oncology is evaluating the safety, efficacy, tolerability, and anti-cancer activity in clinical studies. The Phase 2 ASPEN-06 study, for example, is looking at whether evorpacept—used in conjunction with paclitaxel, trastuzumab, and ramucirumab—could improve outcomes in HER2+ gastric cancer.
EC Orphan Drug Designation
Orphan Drug designation in both the United States and European Union is granted to drugs or biologics that intend to treat, diagnose, or prevent rare illnesses. In the United States (where evorpacept also earned Orphan Drug designation in 2022), rare conditions are defined as those affecting fewer than 200,000 people. For Europe, rare conditions are defined as those affecting fewer than 5 in every 10,000 people. Orphan Drug designation is meant to incentivize the development of drugs in this space to fill an unmet need and get therapies in the hands of the people that need them faster.
For receiving this status from the EC, ALX Oncology now qualifies for protocol assistance, regulatory fee exemptions, and ten years of market exclusivity if the drug is approved.
What is Gastric Cancer?
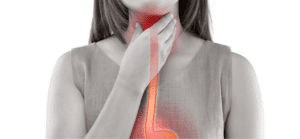
Also known as stomach cancer, gastric cancer forms in your stomach. Your stomach wall has three layers: the mucosal layer (inner), the muscularis layer (middle), and the serosal layer (outer). While cancer can form in any layer, it most often begins in the mucosal layer and spreads outwards. Risk factors include being male, living in a less developed country, being older in age, having gastroesophageal reflux, smoking cigarettes, a high sodium diet, and a family history of gastric cancer. Treatment is often successful if the cancer is identified before it spreads. Chemotherapy, radiation, targeted therapies, and surgery may all be utilized in the care plan.
Symptoms relating to gastric cancer may, but do not always, include:
- Consistent nausea and vomiting
- Bloody stool
- Feeling bloated or full easily
- Chronic indigestion
- Stomach pain
- Dysphagia (difficulty swallowing)
- Jaundice (yellowing of the skin and eyes)
- Unintended weight loss
- Severe heartburn
- Fatigue
- Dark or tarry-looking stools