On October 2, 2025, Novartis announced a significant milestone in the treatment of chronic spontaneous urticaria (CSU): the US Food and Drug Administration (FDA) approved Rhapsido® (remibrutinib) as the first oral, targeted Bruton’s tyrosine kinase inhibitor (BTKi) for adult CSU patients who remain symptomatic despite H1 antihistamine therapy. This approval reported by World Pharma News, introduces a novel, convenient option for patients who previously faced limited choices beyond injectable treatments.
A Breakthrough for CSU Sufferers
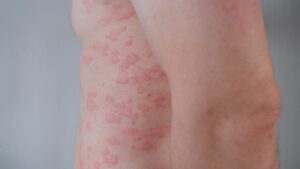
CSU is a chronic, unpredictable skin disorder characterized by recurrent hives and itching lasting six weeks or more without an identifiable trigger. The disease, driven by immune system dysregulation, can severely impact sleep, work, and mental health. First-line antihistamines fail to provide sufficient relief for over half of CSU patients, and while injectable therapies exist, they are underutilized, with fewer than 20% of eligible patients receiving them.
Rhapsido stands out as an oral, twice-daily pill that does not require injections or routine lab monitoring, making it easier to integrate into daily life. By specifically inhibiting Bruton’s tyrosine kinase (BTK)—a protein central to the activation of mast cells and basophils—Rhapsido suppresses the release of histamine and other proinflammatory mediators at the heart of CSU symptoms.
Clinical Evidence and Impact
FDA approval was based on robust results from the Phase III REMIX-1 and REMIX-2 clinical trials, which enrolled patients who continued to experience symptoms despite high-dose, second-generation H1 antihistamines. Rhapsido demonstrated clear superiority over placebo in reducing itch, hives, and overall urticaria activity by week 12. Notably, more patients achieved well-controlled disease as early as week 2, and about a third became entirely symptom-free by week 12. The treatment’s safety profile was favorable, with common side effects (≥3%) including mild upper respiratory symptoms, headache, nausea, and abdominal pain—none requiring lab monitoring.
Transforming Treatment Paradigms
This approval marks the first time a BTK inhibitor has been made available for CSU, offering a patient-friendly oral alternative to injectable therapies. Dr. Mark Lebwohl, REMIX trial steering committee member, emphasized the therapy’s ability to quickly interrupt the immune pathways that trigger CSU, providing rapid relief for a broad spectrum of patients. Dr. Giselle Mosnaim highlighted the importance of treatment options that fit seamlessly into patients’ routines, while Lynda Mitchell of the Allergy & Asthma Network noted that Rhapsido empowers patients to choose therapies that best suit their needs.
Looking Ahead
Novartis has filed for regulatory approval of Rhapsido in multiple countries, including the EU, Japan, and China. The company is continuing to research remibrutinib in other immune-related diseases, underscoring its commitment to advancing immunology and addressing unmet patient needs.