GW Pharmaceuticals is finding out…
Epidiolex® (manufactured and tested by GW Pharmaceuticals) is a leading medical cannabinoid candidate for the treatment of rare, resistant, and early-onset epilepsy syndromes such as Dravet syndrome, infantile spasms, and Lennox-Gastaut syndrome. These rare conditions are extremely difficult to treat, so there is a tremendous urgency to stop seizures and give relief to the children and families affected.
GW has been researching the positive effects of cannabinoid for epilepsy patients for the past ten years, and finally started a series of clinical trials to test the efficacy and safety of Epidiolex® in 2016, hoping to submit a New Drug Application (NDA) to the U.S. FDA within the coming months.
Currently, Phase 3 trials are underway, with the first two of these clinical trials showing significant reductions in specific types of seizures characterized by Lennox-Gastaut syndrome and Dravet syndrome in patients taking Epidiolex® along with their current treatments.
In fact, the median reduction in monthly seizures was a whopping 42%, compared to 17% under placebo.
The CEO of GW is optimistic and headstrong, saying,
“These compelling results make us more determined than ever to make this important new medicine available to patients who suffer from these treatment-resistant childhood-onset epilepsies.”
Let’s keep our fingers crossed that, following the success of these trials, the FDA will approve Epidiolex® as a prescription medication, and the use of cannabis in the medical arena will no longer be seen as a taboo.
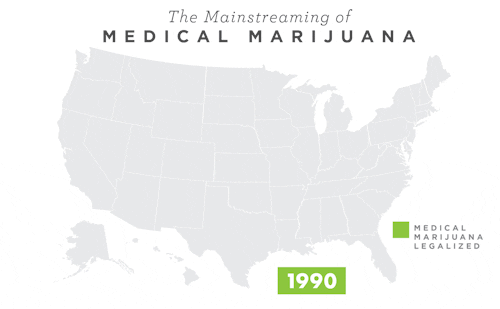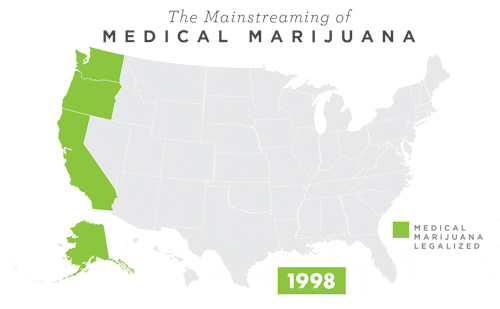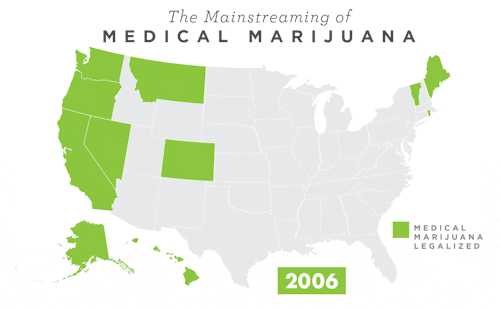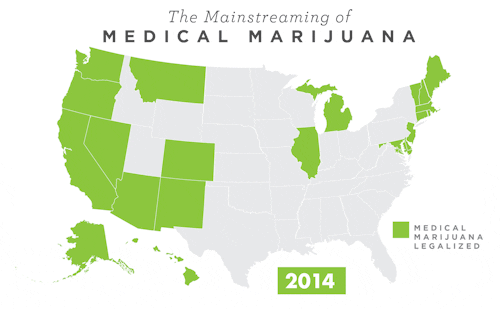
To learn more about Epidiolex®, including GW’s clinical program, click here.
What are your thoughts about ongoing research for rare diseases? Share your thoughts, and your hopes, with the Patient Worthy community!


