According to a story from pm360online.com, the biopharmaceutical company Ultragenyx Pharmaceutical Inc., in collaboration with Kyowa Hakko Kirin Co. Ltd, and Kyowa Kirin International PLC, recently announced that injectable burosumab (marketed as Crysvita™) has gained approval from Health Canada. Burosumab is approved as a therapy for X-linked hypophosphatemia for all patients aged one year and older. Ultragenyx is dedicated to the development of innovative treatments for serious, rare diseases.
About X-Linked Hypophosphatemia
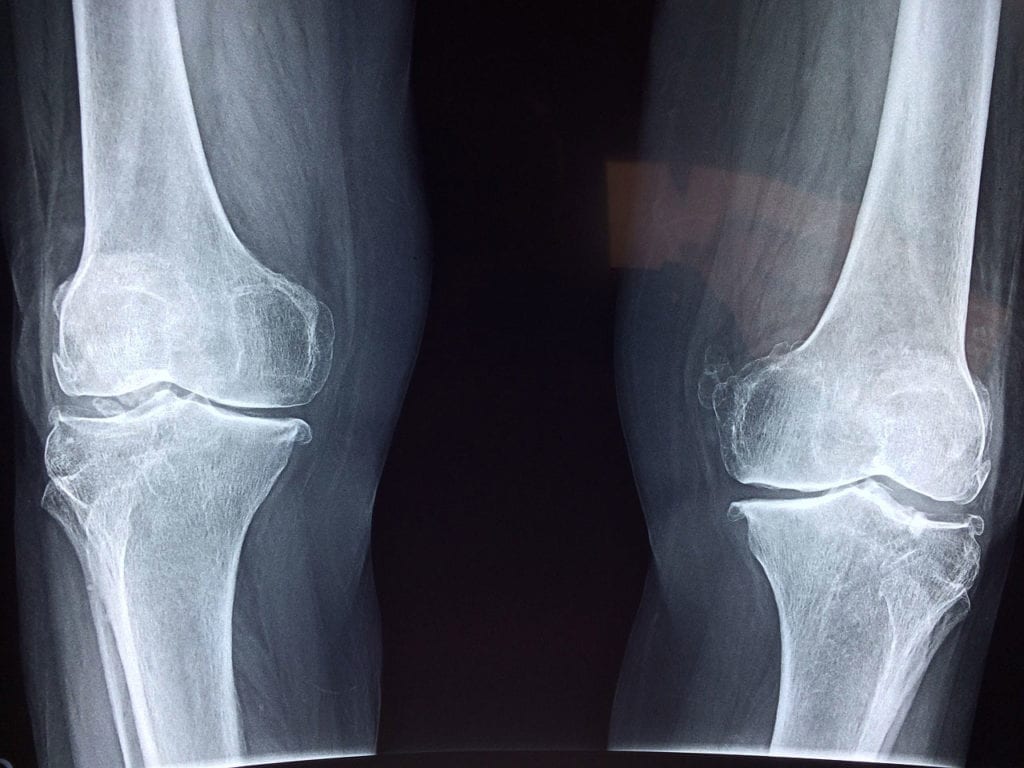
X-linked hypophosphatemia (XLH) is a genetic disorder. It is considered a type of rickets that is distinguished from other types because vitamin D cannot cure it. Rickets is characterized by the abnormal softening of bones which can result in a bow-legged appearance and stance. The disorder is linked to mutations affecting the PHEX gene sequence found on the X chromosome. Symptoms of X-linked hypophosphatemia include soft bones, bone pain, osteoarthritis, teeth problems, and hearing loss. Treatment may include burosumab (as of 2018), calcitriol, phosphate, human growth hormone (HGH), and surgery to correct bowed legs or other bone deformities. The prevalence of this disease is about one in every 20,000 people. Inheritance of the condition is X-linked dominant, which means that men are more likely to express symptoms. To learn more about X-linked hypophosphatemia, click here.
About Burosumab
Burosumab is the first therapy approved specifically for X-linked hypophosphatemia, and it is the only one that targets the underlying mechanism of the disorder. The therapy is also approved for this indication in the US and the EU. It should become available for Canadian patients in early 2019. Burosumab is typically administered under the skin from every two to every four weeks. The drug is capable of reversing the effects of X-linked hypophosphatemia and can restore normal bone mineralization.
The approval of burosumab was hailed as a breakthrough in treating the disorder. As a therapy that has only been introduced to the market in the past year, continuing study is underway to monitor side effects and long term impacts of treatment.
This historic approval is great news for Canadians dealing with the debilitating effects of X-linked hypophosphatemia.