Dr. Cory M. Resnick, Oral & Maxillofacial Surgeon at Boston Children’s Hospital and Assistant Professor at Harvard Medical School, describes the exciting progress he has made on the Prenatal Diagnosis of Pierre Robin Sequence. Interview by Philippe Pakter, chairman of Pierre Robin Europe.

Dr. Resnick, thank you very much for taking the time to speak with us about your work. To begin, can you please tell us about your professional background? Where did you study medicine? What additional specialized training did you receive? And where do you practice medicine today?
In undergraduate school I became very interested in photography. Through the photographic lens, I learned to appreciate the confluence between form and function. I carried this with me through Dental School at the University of Pennsylvania, Medical School at Harvard Medical School, and residency training in Oral and Maxillofacial Surgery at the Massachusetts General Hospital. During my residency, I spent time in all disciplines of surgery and always gravitated toward pediatric surgical specialties. When I was a Chief Resident, I rotated at Boston Children’s Hospital, and this is when I knew that I wanted to focus on caring for children as a career. After spending some time in private practice, I returned to Boston Children’s Hospital to work with my mentor and have been there ever since.
When did you first become interested in Pierre Robin Sequence (let’s call it RS), and why?
Early in my practice at Boston Children’s Hospital, I treated several infants with RS. I was struck by the challenges that children and their families went through during a time that was meant to be filled with joy and promise. I was surprised by the heterogeneity of the disease and the available treatment options. Conversely, I was uplifted by the ability to correct the early breathing problem and bring hope to families. I realized that I could help children and families through a difficult time, allow them to recapture the early childhood years, and work to improve processes and outcomes for future families.
Can you please tell us about your experience treating babies with RS, and your research in RS?
I have very much enjoyed treating babies with RS and watching them grow into happy children. I have seen the hurdles that families affected by this diagnosis experience: grappling with their newborns undergoing anesthesia and surgery, uncertainty of the outcomes, long inpatient hospitalizations, time away from other children and family, lost time from work and resulting financial instability, the prospect of future operations, and more. These difficulties have driven me to try to improve diagnostic and surgical processes and outcomes. I have built a team in my hospital to help families navigate as many of these challenges as possible, and I study outcomes and innovations to improve treatment for future generations.
We would like to speak with you today about the prenatal diagnosis of RS. I am the father of a baby girl born with RS. Our local hospital failed to diagnose our daughter during the prenatal period, in spite of ultrasound examinations which showed severe micrognathia and a severe excess of amniotic fluid. This was in 2017. However, based on the extensive contact we’ve had with other RS parents since then, it seems that our experience was not uncommon. How often do ultrasound teams identify or at least suspect RS during the prenatal period? How often do they miss the warning signs completely?
I am truly sorry to hear about your unfavorable experience with prenatal diagnosis, and my team and others are working hard to improve this for future families. Unfortunately, prenatal diagnosis is very difficult. The biology of human development is amazingly complex – it’s remarkable that so many things go right! Obstetricians and ultrasonographers have a litany of things to look for during these prenatal evaluations, and, traditionally, jaw-related findings have not ranked on high on the priority list. Perhaps the biggest barrier to universal prenatal diagnosis of RS has been the lack of objective measurements that indicate an increased likelihood of the diagnosis; observation of a “small jaw” is very subjective and has not been shown to correlate well with RS.
Before discussing your work on the prenatal diagnosis of RS, I would like to first ask you an important initial question: why should we prenatally diagnose RS at all? What benefits do we gain with a prenatal diagnosis of RS? And what risks do we face when healthcare providers fail to diagnose RS during the prenatal period?
Some of the benefits of prenatal diagnosis relate to medical preparedness: the ability to triage at-risk deliveries to hospitals with appropriate resources and preparation of the delivery team to manage potential airway emergencies. One of the most important benefits, though, is family education. Prenatal knowledge of this diagnosis can help families understand and prepare, which can decrease stress around an already very stressful time. Lack of prenatal diagnosis does not allow for these benefits.
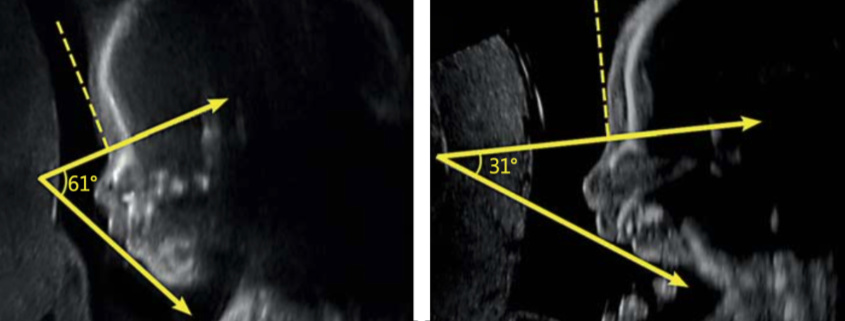
A study published by physicians at Baylor College of Medicine in Texas in 2016 stated that “prenatal diagnosis remains a challenge… no definitive parameters or combination of findings have been described that consistently and accurate assist” with a prenatal diagnosis of RS. The authors of that 2016 study did not carry out experiments, but they did propose an RS screening system which focused on IFA, the Inferior Facial Angle. Can you please explain to us what the IFA is, how it is calculated, and why it is so important?
Yes, IFA is a method of quantifying mandibular (lower jaw) size on an image. The IFA can be applied to either ultrasonography or MRI, though the normal ranges differ between image types. While this measurement alone is not a good predictor of RS, when used in combination with other findings, the IFA plays an important role in prenatal diagnosis of RS.
IFA is calculated by creating a vertical line that touches the forehead, and then a second line perpendicular to the first. The angle made between this second line and a final line drawn through the tip of the upper lip and the front of the chin makes the IFA.
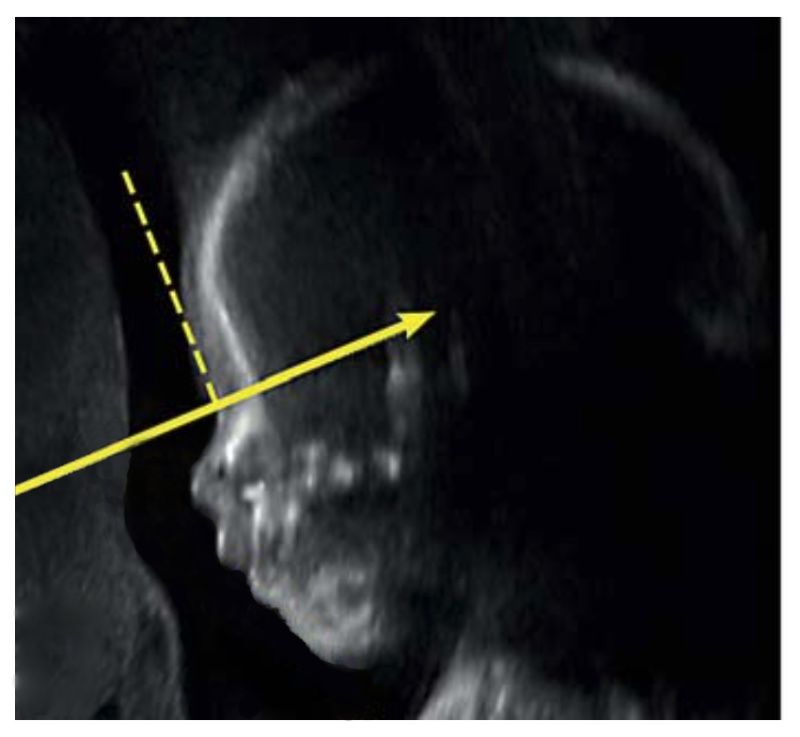
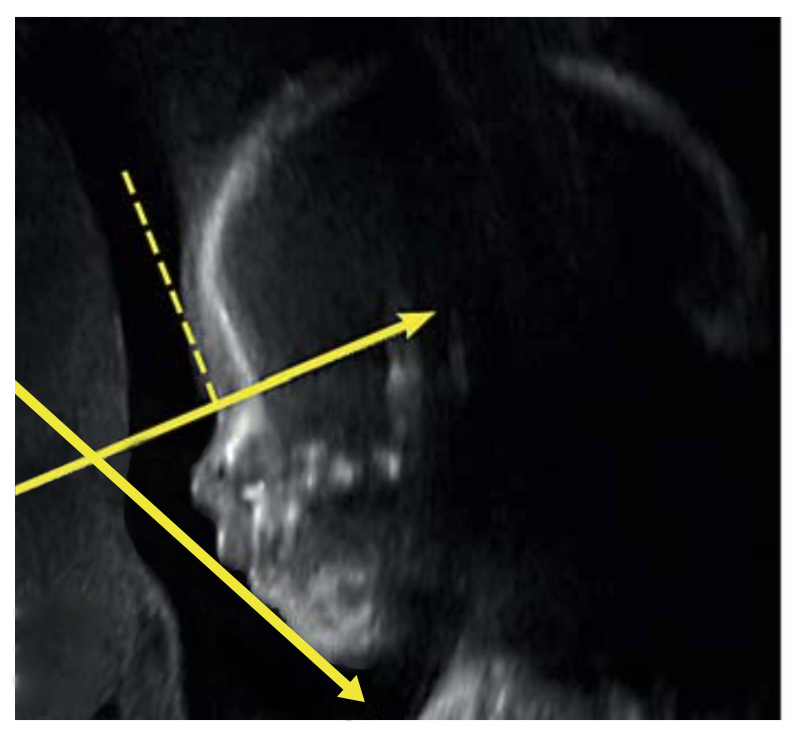
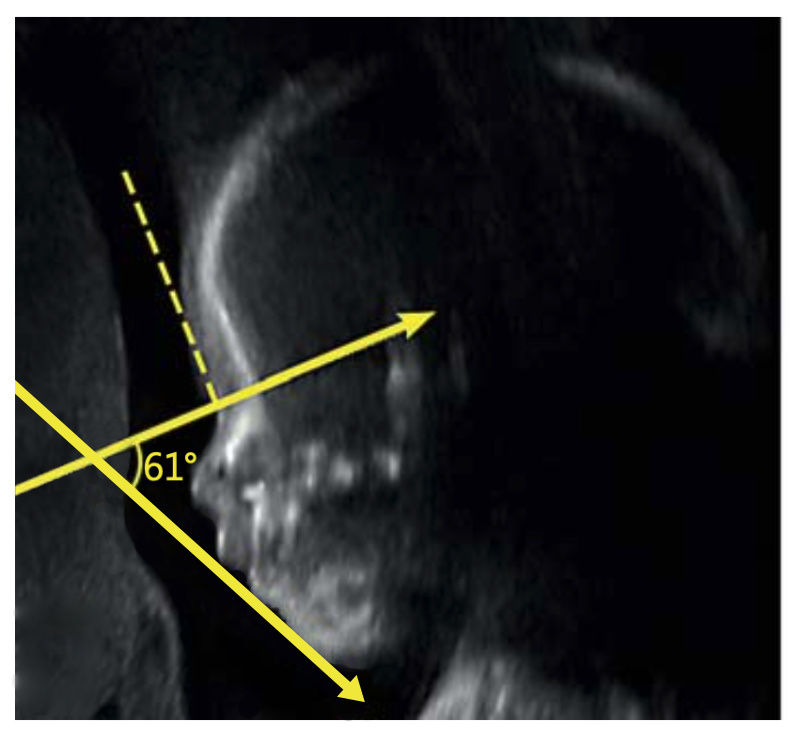
The 2016 study from Texas was based on ultrasound examinations. Two years later, in 2018, you and your colleagues at Harvard Medical School / Boston Children’s Hospital published a very promising study on the prenatal diagnosis of RS. In your 2018 study you carried out a statistical analysis of 162 subjects; based on this analysis, you proposed a prenatal RS screening system which focused on three separate variables. When all three of those variables were present, your RS screening system indicated a 98% probability of RS. This 98% figure is remarkable, but there was one important condition: this screening system was based on and required MRI (Magnetic Resonance Imaging). Most expectant mothers don’t undergo MRI exams; they only undergo ultrasound exams. Why did you focus on MRI?
Yes, as you summarized, prior studies using ultrasonography were not able to reliably diagnose RS. MRI is a much more robust type of imaging that significantly improves visualization of the growing fetus. Like ultrasounds, there is no radiation exposure for an MRI, so it is safe to use during pregnancy. The significant limitation of MRI, though, is that requires special technology and is therefore not available everywhere. Additionally, its expense may limit widespread use.
My goal in studying MRI was to identify factors that could predict RS, and then try to adapt those findings back to ultrasonography. We have recently done some comparisons of our MRI findings in ultrasounds, and, while the ultrasonographic findings are not as predictive as those seen in MRI, we have identified some factors on ultrasounds that can raise concern for RS and prompt referral for MRI confirmation.
Can you describe in layman’s terms the three variables which you identified in MRI which allowed you and your colleagues to predict RS with such a high level of probability, 98%?
We started by evaluating a large series of measurements and other characteristics seen on MRI, and testing which of them, alone and in combination, most closely correlated with babies born with RS. After a robust statistical analysis of all tested variables, we settled on 3 factors that had the highest predictive value for diagnosing RS: Tongue-shape index (TSI), IFA, and presence of a cleft palate.
The TSI is a novel measurement that my group created for preliminary fetal MRI studies, and indicates an abnormal shape of the tongue. We devised this measurement because we know that babies with RS have tongues of normal size but abnormal position. This abnormal position changes the tongue shape and is the direct cause of the airway obstruction seen at birth. In the future, I would love to evaluate the tongue using 3-dimensional imaging and dynamic imaging that demonstrates its changes in shape and position over time (we have started work on this recently), but, for this initial algorithm, I chose to use static tongue measurements to indicate its abnormal position. To do this, I invented the TSI, defined as the tongue height divided by the tongue length.
The other two measurements in the algorithm include the IFA, which we discussed earlier, and the presence or absence of a cleft palate. We know that presence of a cleft palate is not necessary to have the diagnosis of RS, but, because cleft palate occurs in around 90% of babies with RS, this finding is still highly predictive of the diagnosis.
Next, we had to define thresholds for each of these measurements based on a series of MRIs of unaffected and affected fetuses. The total of this work produced an algorithm with very high predictive value for RS.
I have included an example from a prenatal MRI that my group published in 2018.
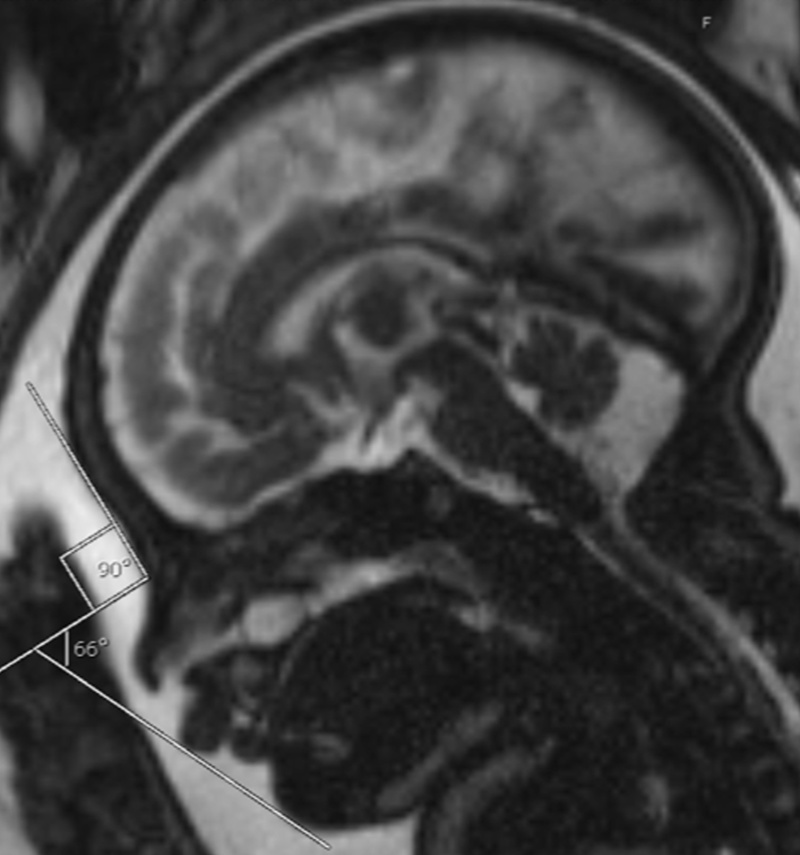
Less than two years later, you and your colleagues published a follow up study… and this 2019 follow up study was based on ultrasound as the standard prenatal imaging tool. Can you tell us about this 2019 ultrasound study, and explain its most important findings?
As we discussed earlier, the goal is to adapt what we have learned from MRI back to ultrasonography, as ultrasounds are ubiquitous in prenatal monitoring. In our first attempt to do this, we had moderate success. We focused on the IFA measurement for this analysis and found a moderate correlation between the ultrasonographic and MRI findings. This means that, for this analysis, the ultrasonographic findings were telling enough to suggest RS, but were not clear enough to make the diagnosis without further evaluation. At this point, use of these ultrasonographic measurements can serve as a screening tool for RS, but an abnormal finding on an ultrasound should still be followed up by a prenatal MRI to confirm the findings. Hopefully, future studies will allow ultrasonography to stand alone in making this prenatal diagnosis.
Is the screening tool which you present in your 2019 ultrasound study something which would be difficult for ultrasound professionals to learn? How much additional time would your screening tool add, if any, to a standard ultrasound examination? Can the same ultrasound equipment be used? Will medical costs go up? Could this screening tool potentially be cost effective?
The ultrasonographic screening is simple and could be easily performed by any experienced ultrasonographer with existing equipment. The challenges will be incorporating another measurement in an already long list of items to be accomplished during those exams, and disseminating the information to the very many centers that perform prenatal ultrasounds.
How exactly will ultrasound teams learn about your proposed screening system? In the context of the US healthcare system, can you and your colleagues, for instance, approach the American Institute of Ultrasound in Medicine, and ask them to include your screening tool in their standard imaging guidelines? Or is this far-fetched?
This kind of approach may work in the future but, with the limited number of measurements and observations that can be made during any single ultrasonographic examination, we will need to define better screening measurements than we currently have before we can realistically expect this to become universal. Working on it!
If I understand correctly, your 2019 ultrasound study is based on the idea that healthcare providers will use ultrasound as the standard, default RS screening tool. If the ultrasound shows an IFA less than 45.5, this will represent a major red flag for RS; an MRI exam will now be warranted. This raises two practical questions. First of all, RS is a rare disease; many healthcare providers and health insurers know little if anything about it. If, on ultrasound, the IFA is less than 45.5, how can parents maximize their chances of getting the follow up MRI exam – of getting the treating physicians to back the MRI, and the health insurers to pay for it?
These are all great questions, but we unfortunately don’t have the answers yet. Currently, every geographic location and insurance plan has independent rules about referring for and covering the costs of fetal MRI. As we improve the data supporting the benefit of these findings, I hope that access to the necessary imaging tools will also improve.
A second practical question: let’s assume that during the ultrasound exam, the IFA was less than 45.5. On the basis of this red flag, the expectant mother requests and hopefully receives authorization to undergo an MRI exam. Ideally, the MRI examiners should now refer to your 2018 MRI study, no? Your 2018 MRI study was very specific; it identified exactly three specific variables which, if taken together, would produce an RS diagnosis with a high level of probability. But what if the MRI examiners are not familiar with your 2018 MRI study – what happens then? Are we now facing more training and implementation challenges, this time on the level of MRI?
Perhaps yes, but I would expect that, if a patient is referred for a specific test with a well-defined question (concern for RS based on ultrasonographic measurement of IFA), then the referral center would already be or seek to become knowledgeable about the best available confirmatory test. This is how our diagnostic processes improve as we learn more about disease.
What do you envision in terms of local and international uptake of your 2019 ultrasound screening tool? Do you believe that healthcare systems will be receptive? Or do you think we’re looking at many more years of missed RS diagnoses during prenatal ultrasound exams in America, in Europe, and throughout the world?

I see the current ultrasonographic screening tool as only a first attempt at making the prenatal diagnosis of RS ubiquitous. So far, I do not expect that it is strong enough to be adopted universally, particularly in resource-poor areas. We are continuing to learn and refine our techniques, however, and I am hopeful that we will someday be able to offer this screening to every expectant mother.
Let’s talk about the next steps – proving that the RS screening tool which you have developed is consistent, accurate, reliable, and feasible to implement. Can you begin by explaining to us the difference between a retrospective clinical trial, and a prospective clinical trial? And can you explain to us the advantages and disadvantages of both, in the context of RS?
All of the work I have done has been retrospective. This means that I have looked back on prior patients and images that were obtained for routine care and have used those existing data to draw conclusions. This is typically how initial discoveries are made in medicine. However, to prove that one approach is better than another, a prospective trial is necessary. This means that protocols are developed in advance, patients are randomly assigned to one group or another, and the outcomes are carefully compared. This type of trial is difficult to do in RS, and, moreover in prenatal diagnosis of RS, because the condition is rare and there are few institutions with the technology, expertise, and patients to compare. Additionally, these types of trials require large funding and dedicated personnel. Nonetheless, a multi-institution prospective study remains a future goal for this work.
Have you and your colleagues already begun planning prospective clinical trials, to validate your RS screening tool? Would you be open to collaborating with other hospitals? Or is everything on hold now, as a result of COVID-19?
Yes, we have discussed collaborative prospective trials with other institutions, and I am certainly interested and excited to work with other centers. COVID-19 has certainly delayed this process, but we will continue to work toward these goals in time.
Would you like to share with us anything else about your work?
I appreciate the opportunity to share with your group, and I truly hope that my work and that of many others can have a positive impact on children and families.
Dr. Resnick, as an RS parent, I sincerely thank you and your colleagues for your efforts on behalf of RS babies; thank you for the substantial contributions you have already made. We look forward to receiving updates from you in the future about your valuable work. Thank you for taking the time to speak with us.
Click here to learn more about the activities of Pierre Robin Europe.


