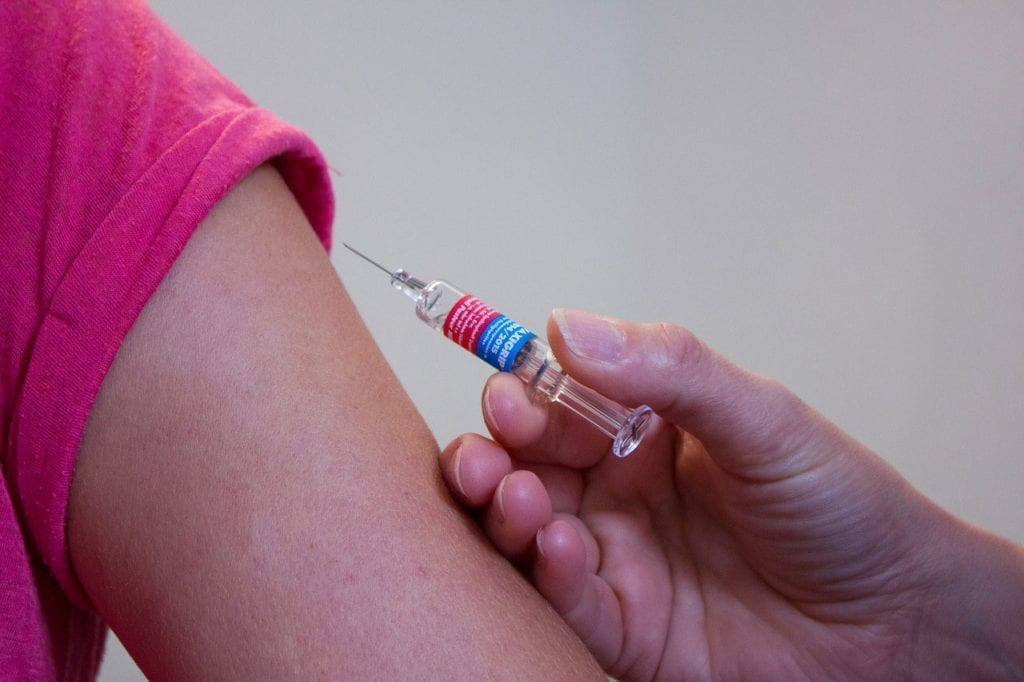
Prior to February 2023, there were no FDA-approved treatment options for non-central nervous system manifestations of alpha-mannosidosis. But in mid-February 2023, Chiesi Global Rare Diseases shared via news release that the FDA approved Lamzede (velmanase alfa-tycv) for both adults and children with alpha-mannosidosis. Lamzede, a recombinant form of human alpha-mannosidase, is now the first FDA-approved enzyme replacement therapy (ERT) available for this indication. It is administered via infusion.
Lamzede may cause hypersensitivity reactions in individuals with alpha-mannosidosis. Because of this, pretreatment with corticosteroids, antipyretics, or antihistamines may be recommended. Potential Lamzede side effects may include anaphylaxis, fever and chills, nausea and vomiting, tremors, pink eye, facial swelling, rashes, low blood pressure, cyanosis (bluish tinge to the skin and lips), hives, cough, headache, and muscle aches. Learn more about Lamzede.
An Overview of Alpha-Mannosidosis
Alpha-mannosidosis is a rare and progressive genetic lysosomal storage disorder caused by MAN2B1 gene mutations. Normally, this gene encodes for the production of alpha-mannosidase, which breaks proteins into smaller pieces. When the body doesn’t have enough of this enzyme, these proteins accumulate in cells to toxic levels, leading to symptoms and organ damage. Alpha-mannosidosis is delineated into three subtypes. Type 1 is mild, slow-progressing, and appears after age 10; individuals with this subtype often do not have muscle issues or skeletal abnormalities. Next, type 2 is considered moderate, slow-progressing, and appears before age 10. This is the most common subtype and also does not present with muscle or skeletal issues. Finally, type 3 is the most severe form and usually appears within the first year of life; this form causes rapidly worsening symptoms and may lead to early death.
Given the three subtypes, symptoms may differ from patient to patient. On a broader scale, potential symptoms may include:
- Hearing loss
- Muscle weakness
- Delayed motor skill development
- Intellectual disability
- Increased risk of infections
- Hydrocephalus
- Immune dysfunction
- Enlarged tongue
- “Coarse” facial features
- Difficulty with coordination and movement
- Skeletal abnormalities such as reduced bone density, deformed vertebrae, joint deterioration, and bowed legs
- Speech impairments
- Cataracts
- Depression and/or anxiety
This is not an exhaustive list of symptoms or characteristics. Treatment for alpha-mannosidosis is symptomatic and supportive. Therapeutic interventions may include enzyme replacement therapies, hearing aids, shunts, surgery, physiotherapy, mobility aids, antibiotics, bone marrow transplantation, and speech therapy.


-239x300.jpg)


