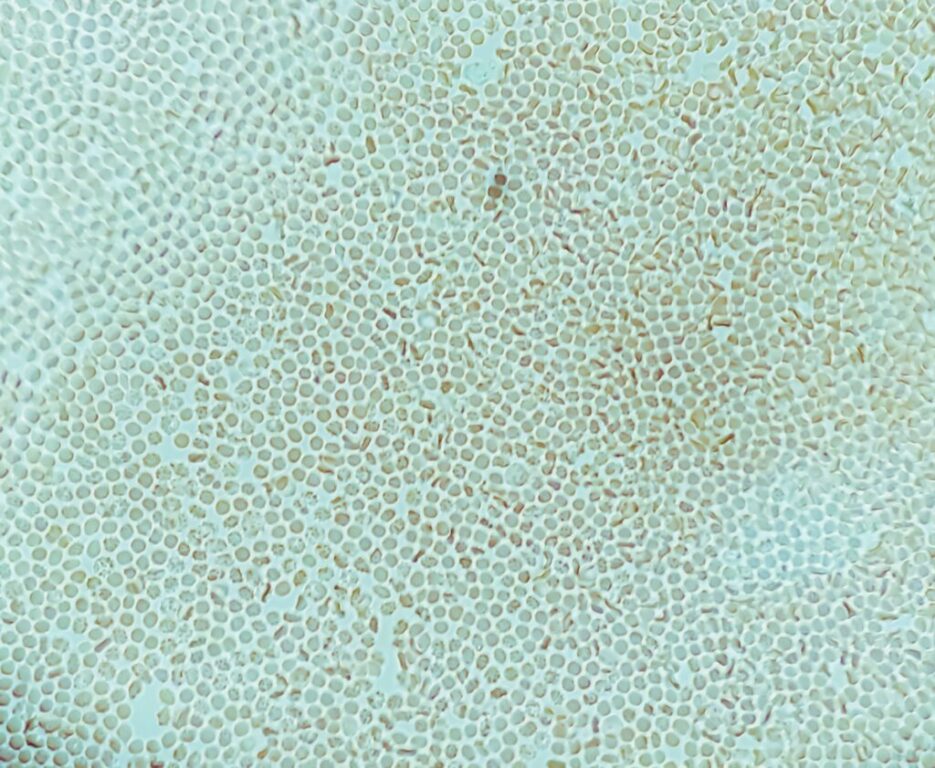
Sickle Cell, an inherited disease, involves crescent shaped red blood cells that are the “vehicles” containing hemoglobin, a protein responsible for carrying oxygen throughout the body.
For almost a year after FDA approval of two sickle cell anemia gene therapies, doctors at the Medical Center in Boston report that only two patients have received an infusion. Comparatively, five dozen patients have begun the personalized treatment process illustrating the length of time required to produce the two therapies.
The drugs are Casgevy, developed by Vertex Pharmaceutical and Lyfgenia developed by Bluebird bio. The expectation was to have infused a minimum of four or five patients by November 2024. This goal has not been realized.
Dr. Martin Steinberg admits that expectation may have been overly optimistic. Dr. Steinberg and his associates planned to infuse the therapy in a minimum of five patients with either of the therapies in 2024 and perhaps a dozen the following year.
Although a slow start is somewhat common in producing new medicines, especially complex products, it is important to recognize these two medicines for their curative potential. Neither drug is a direct fix for gene mutations that result in warping of red blood cells. The genetic engineering of the patient’s own stem cells improves the functioning of the cells obtained from the stem cells. Both treatments, in most cases, relieved the pain most patients with severe disorders from SMA generally experience. Therefore, many of these patients discontinued blood transfusions and hospital stays.
Vertex offers a program, Vertex Connects, that supports patients receiving Casgevy therapy. Casgevy-Lyfgeniai is a one-time infusion, but it requires years of follow-up. The latest data that was presented recently at ASH indicated that both therapies were meaningful and durable. The process requires a few visits to the hospital and the period from the initial evaluation to an infusion may involve a one-year time frame.
Preparatory chemotherapy using the drug busulfan, is somewhat taxing on the patient and may cause infertility. Also, a warning on Cassgevy Lyfgeniai’s label suggests the risk of blood cancer.
Source: https://www.biopharmadive.com/news/sickle-cell-gene-therapy-slow-uptake-casgevy-lyfgenia/734938/