The Oley Foundation
The Oley Foundation is a national non-profit organization, founded in 1983. They aim to “enrich the lives of patients dependent on home intravenous nutrition (parenteral) and tube feeding (enteral) through education, advocacy, and networking.” They are a “resource for consumer’s families, clinicians and industry representatives, and other interested parties. The Foundation gives members the tools and confidence they need to manage their complex therapy and enables them to achieve normalcy in their lives.”
Condition Awareness & Advocacy
Here is a list of conditions this partner raises awareness and advocacy for:
Resources & Support
Patient Worthy Posts on Gastroschisis, Short bowel syndrome, Systemic Scleroderma, Gastroparesis

Celebrate Gastroschisis Month with Jeanie and Her Gastroschisis Baby
The incidence of gastroschisis has doubled over the past 20 years. Read “Jeanie’s Gastroschisis Baby” and learn from a brave, young first-time mom about discovery

Familial Adenomatous Polyposis: Jenny’s Rare Patient Story
Written by Jenny, Life’s a Polyp I come from a long family line of individuals with familial adenomatous polyposis (FAP). My family though would learn

A Phase 3 Study is Being Designed to Evaluate Vurolenatide for Short Bowel Syndrome
Clinical trials are a crucial part of learning more about certain diseases, as well as better understanding the impact of various therapeutic options. For
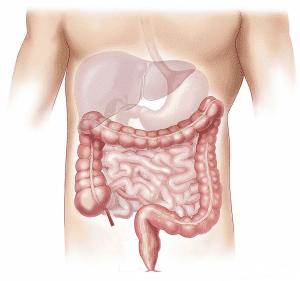
Preliminary Data Available on Vurolenatide for Short Bowel Syndrome
Medical research and clinical trials are extremely important tools in learning more about specific diseases, as well as understanding and evaluating potential treatments. For

Rare Classroom: Gastroschisis
Welcome to the Rare Classroom, a new series from Patient Worthy. Rare Classroom is designed for the curious reader who wants to get informed on

NIH Grants Funding for Short Bowel Syndrome Research
One of the largest problems that stands in the way of rare disease research is funding. It can be difficult to raise the enormous amounts







