Discovery Phase Drugs for Orphan Eye Diseases
IVMED-100- 80- 90 are currently without generic drug names. They are under development for the treatment of orphan eye diseases by iVeena, LLC who has 6 pipeline products spanning the discovery and preclinical stages of development.

This is just huge! Eyes are really important, duh. More R&D!
Achromatopsia, Best’s Disease, also known as Vitelliform Macular Dystrophy, Choroideremia, De Morsier’s Syndrome, Keratoconus and non Non-24-Hour Sleep-Wake Disorder are just a few rare diseases that affect the eyes.
Most rare eye diseases are often inherited and progressive. The NIH’s National Institute of Health reports the following rare diseases can affect the eye as well:
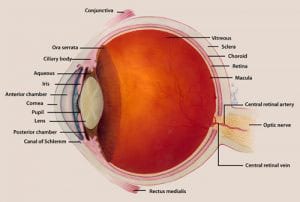
- Anophthalmia and Microphthalmia
- Bietti’s Crystalline Dystrophy
- Behçet’s Disease
- Idiopathic Intracranial Hypertension
- Retinitis Pigmentosa (from Laurence-Moon-Bardet-Biedl Syndrome)
- Retinoblastoma
- Stargardt Disease
- Usher Syndrome
- Uveal Coloboma
The following 3 therapeutic candidates are in discovery (proof of concept) stage of development for therapy area, ophthalmology with orphan disease indications:

IVMED-100
IVMED-80
IVMED-90
Why Patient Worthy is keeping an EYE on iVeena, LLC
iVeena, LLC is a Moran Eye Institute spin-out company providing intraocular drug/device delivery to treat major eye diseases (like AMD- age related macular degeneration, or the leading cause of blindness in the United States). iVeena is a privately held startup that according to our research launches its solutions into the market via strategic channel partnerships that are focused on drug device combinations.
We want to know more and so should you. Reach out to [email protected].