CONTENT WARNING: Severe skin wounds and damage
Working in rare disease is always hard- It means working in suffering and grief, but even with all our experience, we had to walk outside and breathe when meeting kids with EB.
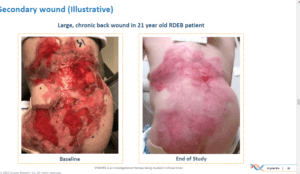
Good news does sometimes come, and this week, following an interview with Dr. Amy Paller, Professor and Chair, Professor of Pediatrics and Director of the Skin Disease Research Center at Northwestern University’s Feinberg School of Medicine, we feel certain that good news has come for those with EB. Dr. Paller has been treating kids with EB from birth and she can see after working with them in the GEM-3 trial, that this innovative treatment is hijacking the pain and the blistering, reducing the itching and the scarring and may-be able to make a difference in the inflammation. What does it mean for a kid to be able to walk, play live without creating weeping sores ? it is life changing!
Krystal Biotech has just announced results from their Phase 3 trial called GEM-3 and the results were all positive! This trial investigated bermemagene geperpavec (B-VEC), or VYJUVEKTM as a potential therapy for dystrophic Epidermolysis Bullosa (DEB or dystrophic EB).
This is the very first topical gene therapy which is noninvasive and redosable for DEB. It is also the only genetic treatment for this condition to be investigated in a Phase 3 trial.
Dystrophic EB
DEB is a rare disease which impacts the mucosal tissue and the skin. It occurs as a result of a mutation in COL7A1. Without a properly functioning COL7A1, COL7, a collagen protein, does not form correctly. A healthy COL7 works to attach the dermis to the epidermis correctly. In DEB, these layers of skin are not properly attached, leading to very fragile skin.
DEB patients face frequent wounds in the skin which can become infected. They also face fibrosis, fusing the fingers or the toes together. Additionally, DEB patients are at a high risk of developing squamous cell carcinoma, a potentially fatal condition.
VYJUVEKTM
This therapy is a topical gene therapy which is non-invasive. It works to deliver two COL7A1 copies to patients with DEB when applied directly to the wounds.
This therapy is so novel because it treats DEB at a molecular level. The skin cells are provided a template to create a normal version of COL7. As such, the cause of the disease is treated as opposed to simply its symptoms.
Orphan Drug Designation has already been granted to VYJUVEKTM by both the EMA and the FDA. Additionally, the FDA has granted the treatment Regenerative Medicine Advanced Therapy, Rare Pediatric Designation and Fast Track Designation. Further, the EMA granted it PRIority MEdicines eligibility.
GEM-3 Trial
This trial was a randomized and double blind placebo-controlled investigation. Its aim was to evaluate both the safety and the efficacy of VYJUVEKTM as a treatment for DEB. There were a total of 31 patients who were between the ages of 1 and 44. These patients were distributed across 3 trial sites.
For every patient, doctors documented one primary wound pair. These wounds were randomized so that one would receive placebo and one would receive a once a week application of the investigative therapy. This once a week application continued until the wounds closed, and began again if the wounds reopened.
In addition to these wounds, secondary wounds were also treated with the therapy each week.
The primary endpoint was complete wound healing at the 6 month mark. Secondary endpoints included wound healing at 3 months and pain.
30 days after the last treatment, all patients returned to the trial location to be evaluated for safety. All patients also were given the opportunity to begin an Open Label Extension study. At this point, other patients who were not initially included in the trial were also given the opportunity to join the extension study.
Notable Findings
Some of the most notable findings from this clinical trial are as follows-
- 67% of all of the wounds which were treated with the novel therapy achieved complete wound healing by the 6 month.
- 22% of all of the wounds treated with the placebo achieved complete wound healing at 6 months.
- 71% of all of the wounds treated with the therapy achieved complete wound healing at 3 months.
- 20% of all of the wounds treated with the placebo achieved complete wound healing at 3 months.
- There was a statistically significant difference between the wounds which achieved healing in the treatment group and the placebo group.
- The treatment was found to be safe and well tolerated.
- There were no serious drug-related AEs in the trial.
- No patients stopped the trial due to safety concerns.
- There was one mild drug related AE.
- The findings were consistent with the Phase 1/2 trial.
Looking Forward
Krystal is planning on filing a BLA with the FDA in the first half of next year. Following this, they will submit a MAA in Europe. The goal is ultimately to get this therapy to DEB patients as soon a possible.
The company is leveraging its gene therapy platform to pursue treatment for other diseases like cystic fibrosis, Netherton syndrome, and TGM1-deficient ARCI.
Researchers are extremely pleased by the positive results from this trial and are hopeful about bringing this treatment to patients soon.
Have you suspected EB but lacked genetic testing. Krystal Biotech is offering enhanced access to genetic testing. Check it out here: https://www.genedx.com/Krystal/Krystal-Decode-DEB
You can read more about this study and its findings here.
Celebrities Courtney Cox and Kaley Cuoco are also supporters of the EB community. Check out Kaley’s advocacy here, and you can learn more about Courtney’s support here.


