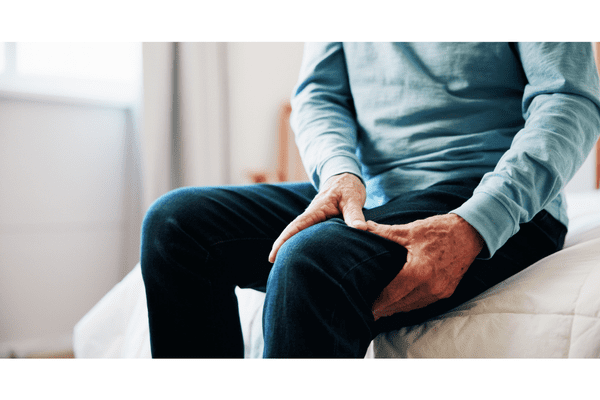
As reported by Clinical Trials Arena, Axsome Therapeutics has begun dosing patients in its FORWARD Phase III clinical trial, a pivotal study designed to evaluate the investigational therapy AXS‑14 (esreboxetine) for fibromyalgia. The condition, which affects an estimated 17 million people in the United States, is marked by chronic widespread pain along with symptoms such as sleep disruption, cognitive difficulties, fatigue, and mood impairment.
A Randomized Withdrawal Study Design
The FORWARD trial uses a randomized withdrawal approach, beginning with a 12‑week open-label phase in which all participants receive AXS‑14. Patients who show meaningful symptom improvement will then enter a double-blind stage, where they are assigned to continue AXS‑14 or switch to placebo for up to 12 additional weeks. The primary outcome measure is the time from randomization to the return of significant symptoms, offering insight into the drug’s ability to sustain therapeutic benefit.
Addressing an Unmet Medical Need
Although fibromyalgia is common and can severely impact daily functioning, available treatments often fall short. Many patients discontinue therapy within a year due to insufficient relief or intolerable side effects, underscoring the demand for new therapeutic options.
AXS‑14, a selective norepinephrine reuptake inhibitor, is the SS-enantiomer of reboxetine and is currently being studied specifically for fibromyalgia. The drug has not yet received FDA approval.
Axsome’s Growing CNS Portfolio
Axsome continues to expand its focus on central nervous system disorders. The company already markets FDA‑approved treatments for major depressive disorder, excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea, and migraine. Additional late-stage programs target a range of neurological and psychiatric conditions affecting more than 150 million Americans.
In 2025, Axsome reported that its Phase III FOCUS trial of Sunosi (solriamfetol) for attention‑deficit hyperactivity disorder achieved its primary endpoint, demonstrating a 45% reduction in ADHD symptoms—further highlighting the company’s momentum in CNS therapeutic development.