In the United States, during the drug development and approval process, drug developers are able to request Fast Track designation. This process is designed to facilitate and expedite the development and review of therapies designed to treat serious conditions. In a late June 2022 news release from biopharmaceutical company Paratek Pharmaceuticals, Inc., the company shared that its product NUZYRA (omadacycline) had received Fast Track designation for the treatment of Nontuberculous Mycobacterial (NTM) lung disease caused by Mycobacterium abscessus (MAB) and Mycobacterium avium complex (MAC).
What is NUZYRA?
The prescribing information for NUZYRA explains that the treatment is a:
tetracycline class antibacterial indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) [or] community-acquired bacterial pneumonia (CABP).
NUZYRA is orally administered, but can also be given intravenously. This antibiotic is taken once daily and has the ability to help those with previously drug-resistant bacterial infections.
Fast Track Designation: An Overview
The FDA explains that the purpose of Fast Track designation is to more quickly and effectively get therapies into the hands of patients in need. This designation is granted to treatments which either treat serious, rare, or life-threatening conditions, or to those which fill an unmet need. According to the FDA:
Filling an unmet need is defined as providing a therapy where none exists or providing a therapy which may be potentially better than available therapy. If there are available therapies, a Fast Track drug must show some advantage, such as showing superior effectiveness, avoiding serious side effects, improving the diagnosis of a serious condition, [or] decreasing a clinical significant toxicity of an available therapy.
Receiving Fast Track designation also imbues the drug developer with benefits such as more frequent meetings and communication with the FDA, Accelerated Approval and Priority Review eligibility, and Rolling Review.
Studies on NUZYRA
In October 2021, Paratek Pharmaceuticals launched a Phase 2b clinical trial to determine the safety, efficacy, and tolerability of NUZYRA for MAB-related NTM lung disease. Altogether, 75 individuals will take part. More data will be available in the future.
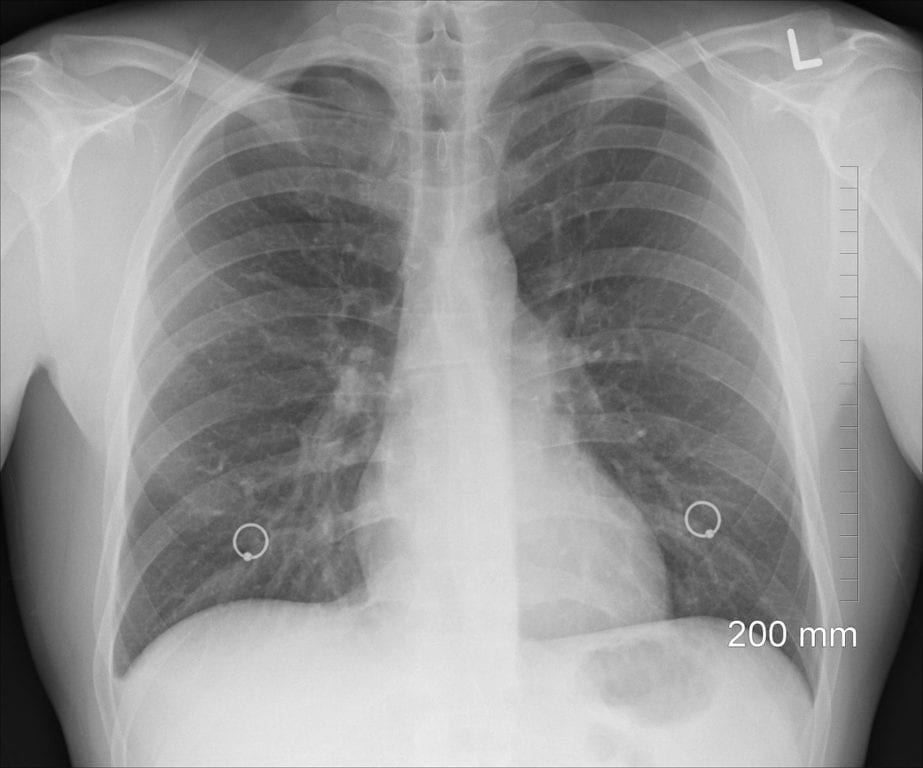
About Nontuberculous Mycobacterial (NTM) Lung Disease
Nontuberculous mycobacterial (NTM) lung disease is an infectious disease caused by certain types of mycobacteria, or harmful germs that are found in our natural environment. Many people come into contact with mycobacteria daily, as these mycobacteria can be found in soil and water. However, most people do not get sick from these mycobacteria. Those with pre-existing health or pulmonary conditions – such as COPD, cystic fibrosis, or bronchiectasis – are at a heightened risk of developing NTM lung disease. The risk also increases with age. Symptoms can include:
- Shortness of breath and/or difficulty breathing
- Unintended weight loss
- Severe, persistent cough which may produce blood
- Fever and night sweats
- Excessive mucus production
- Fatigue
- Frequent or recurrent respiratory infections