In the world today, there are 30 million Americans affected by 7,000 different forms of rare diseases, all in need of treatment. But because the diseases are so rare, treatment for them can be equally rare. According to the National Organization for Rare Disorders (NORD), since the passing of the Orphan Drug Act (ODA) and the Orphan Drug Tax Credit (ODTC) in 1983, over 500 orphan drugs have been approved by the FDA.
To give you some background: An orphan drug is a pharmaceutical treatment that is specifically designed to treat rare conditions–conditions that affect fewer than 200,000 patients in the United States. The ODTC was designed as part of the ODA to promote research spending on orphan drugs. Before the enactment of the ODTC, drug developers were reluctant to invest in developing new treatments because of the small patient population, the subsequent lack of funds, and the number of regulatory barriers. For this reason, only 40 drugs to treat rare diseases were approved in the United States before 1983, and a mere two new orphan drugs were developed annually. And without treatment, the death of patients with rare diseases steadily increased.
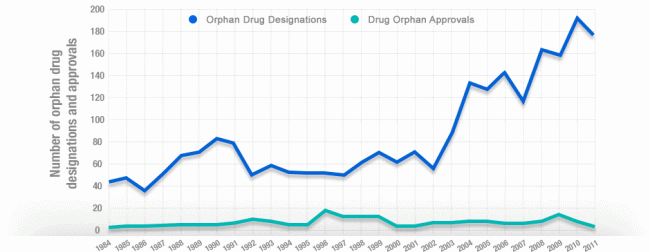
What the ODTC does is provide drug developers with a tax credit for 50 percent of qualified clinical trial costs, thus encouraging the development of orphan drugs. With greater chance of reward, drug developers are willing to take greater risk. Over the next 10 years, if the ODTC were revoked, it is estimated that 33% fewer new orphan drugs would be developed. Here’s hoping that doesn’t happen! For now….these new drugs are saving lives.
And that includes the life of Joe Ellenbergerm, a former high school wrestling champion and two-time NCAA All-American wrestler. Joe was diagnosed with Paroxysmal Nocturnal Hemoglobinuria, a rare blood disease, in 2009. The disease can have serious consequences, including chronic kidney disease and blood clots. Doctors told him he would not live past the age of 30. But after settling into a treatment routine with an orphan drug, Joe is back to wrestling and has dreams of becoming a professional martial artist. See how orphan drugs played a key role in the up and down journey of a young girl battling Muckle-Wells.
With the approval of orphan drugs on the rise, others just like Joe can now begin looking ahead toward a potentially brighter future.
Read the full white paper published by RareDisease.org.
———————————-
- Lowered the cost of medication
- Spurred research and development
- Improved quality of life and increased productivity
- Lessened the rate of deaths from rare diseases in the United States
- Pushed out over 500 new orphan drugs






