According to a study published in PLOS Medicine, only around half of pragmatic or ‘real-world’ trials collect data as reported by patients or involve patients as partners in research. This can include measures such as quality of life, disease burden, or the patient experience during the trial in general. This study was organized by the University of Ottawa’s Shelley Vanderhout. These are not clinical trials, but trials that evaluate a drug’s impacts under real-world conditions after they have been approved.
Patient Input is Essential
In order to be effective, it’s more or less critical for these studies to incorporate at least some input from patients themselves, such as quality of life or experiences of symptoms such as pain. Additionally, such trials should include patient engagement, which allows patients to be partners with researchers during the study as opposed to mere subjects.
About The Study
This observational study evaluated a total of 415 pragmatic/real-world trials that saw publication between January of 2014 and April of 2019. Along with finding that only about half of them incorporated patient reported outcomes, and only nine percent included public or patient engagement. The analysis found that trials conducted in Europe had a greater likelihood of including patient reported outcomes. Trials in low or middle income countries were less likely, as were trials that mostly included elderly or child patients.
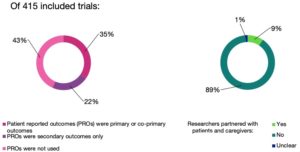
These findings are probably a bit disappointing to patient readers, and these results indicate that these trials still have a lot of room to improve if they are truly going to accomplish the goals that they are allegedly aiming to achieve. The researchers in this study urge research journals, institutions conducting trials, and funding agencies to place a much stronger emphasis patient engagement and outcomes. A quality trial must have a patient centered approach.
In recent years, the healthcare, pharma, and medical industries have made a concerted effort to change their language and tone in a manner that centers patients and their experiences. However, the aspirational, patient-focused language used in these fields often far outstrips the reality, in which patients can still feel left behind. The study authors conclude:
“Patient-reported outcomes can provide a window into patient experience and well-being, but we found that they are not routinely measured in clinical trials which aim to answer questions about patient care in everyday life. It’s worth considering the potential benefit of partnering with patients and caregivers when designing this type of research, to ensure the outcomes that are important to them are captured.”





