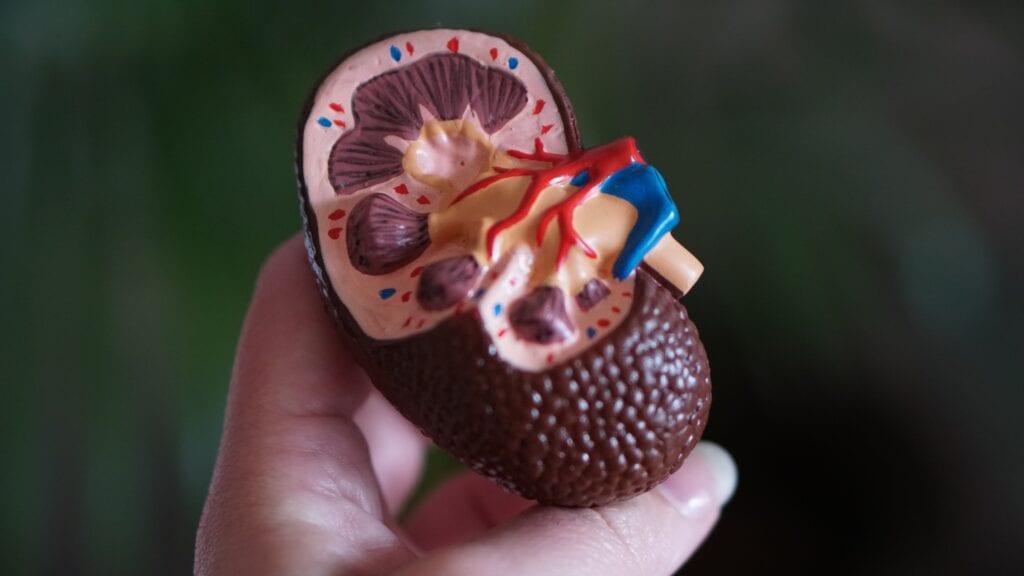
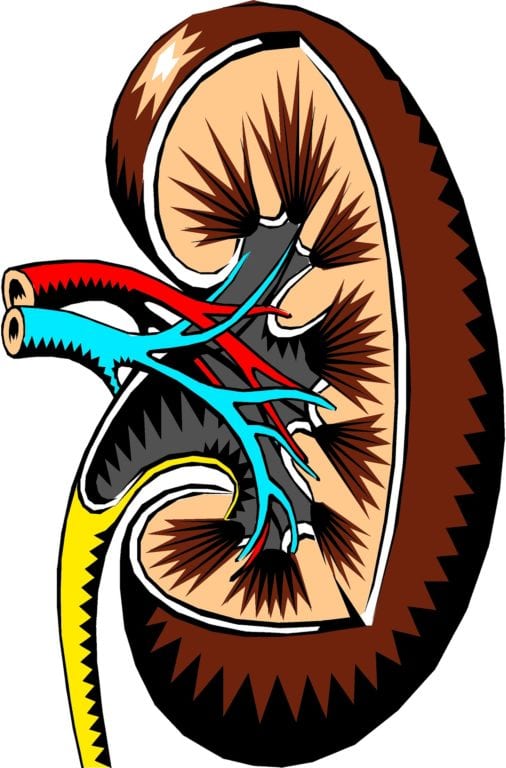
FDA Approve Drug That Targets Root Cause of Life-Threatening Kidney Disease Affecting Children and Young Adults
Iowa University researchers at its Children’s Hospital led the effort that found a new target. According to the Iowa Carver College of Medicine, to date, efforts by researchers focused on…