Because of the current global situation involving coronavirus (COVID-19), many medical organizations have canceled or postponed their annual meetings. The American Academy of Allergy, Asthma, and Immunology (AAAAI) is one such group.
However, despite its cancellation, more than two dozen abstracts on hereditary angioedema (HAE) were ready to be presented. Check out the multitude of abstracts on HAE – and other medical insights – here.
What is Hereditary Angioedema?
Hereditary angioedema (HAE) is an autoimmune disorder caused by a genetic defect. The C1-inhibitor does not work or function well, leading to excess amounts of bradykinin, a protein that causes inflammation. Those with HAE experience “attacks,” where sudden swelling under the skin can happen. Find out more about HAE.
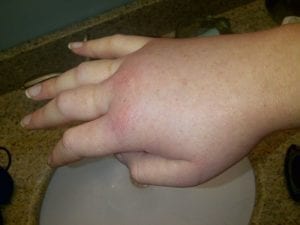
Hereditary angioedema is normally treated by allergists. As initially discussed in Medpage, this is because the symptoms experienced during an attack are reminiscent of severe allergic reactions.
Findings
Cluster Attacks (Abstract 334)
In Ulrich Strassen’s “Etiology and Predictors of Hereditary Angioedema Cluster-Attacks Despite Pharmaceutical Treatment,” he attempted to understand the risk factors and predictors of HAE attacks. HAE attacks may repeat over a period of a few days, or a specific amount of time. Patients on medication may also experience repeated cluster attacks.
His research focused on 15 patients treated in a period of 3 years. The HAE attacks occurred within one week of receiving treatment. Strassen discovered that:
66% of all cluster-attacks were caused by exogenous stimuli (36% due to psycholog- ical stress, 27% due to physical stimuli, and 4% due to menstruation, 1% due to infections).
Additionally, patients who took on-demand treatment were more likely to have a repeated attack sooner. Strassen recommends the use of prophylaxis to treat cluster attacks, which should also cut down on redosing needs.
Lanadelumab
Lanadelumab is a monoclonal antibody drug that was approved for the treatment of HAE in 2018. The drug was given orphan drug designation by the FDA. Many of the abstracts on HAE submitted for discussion consider the results of lanadelumab’s phase III registration trial.
Efficacy of Lanadelumab (Abstract 316)
Paes, Craig, Bernstein et. al’s abstract “Efficacy of Lanadelumab in Hereditary Angioedema Patients With and Without Prior Long-Term Prophylaxis Use: Interim Results From the HELP Open-Label Extension Study” found that long-term treatment with 300mg of lanadelumab greatly reduced patient HAE attack rates.
HAE Attack Rate Reduction (Abstract 318)
Banerji, Riedl, Nurse et. al examined the OLE and phase 3 of the HELP trial. Rollovers, or patients who had already completed the double-blind study, received 300mg of lanadelumab on the first day of this study, then 300mg every two weeks following their first hereditary angioedema attack. New patients received 300mg of lanadelumab bi-weekly. Monthly HAE attack rates were reduced by up to 90%.
Long-term Usage Efficacy and Patient Safety (Abstract 319)
Busse, Tachdjian, Longhurst et. al sought to explore the long-term impact of lanadelumab for younger patients between the ages of 12 and 18. They wanted to test both its efficacy and safety to determine how it worked, and how it was tolerated, over time.
The researchers found that HAE attack rates declined about 90%, comparable to the Banerji, Riedl, Nurse et. al study. However, eleven patients experienced treatment-emergent adverse events (TEAEs). While the researchers do note that none of these were serious or severe, they do not express what the TEAEs are. The study ultimately concludes that lanadelumab is well-tolerated for long-term adolescent use. However, with approximately half of the study participants experiencing TEAEs, researchers may want to look into how to reduce these in the future.
Status of Extended Treatment Patients (Abstract 322)
Riedl, Johnston, Lumry et. al sought to examine how many patients were HAE attack free during the extension of the lanadelumab study. They followed a total of 212 patients. Around 80% receiving 300mg of lanadelumab bi-weekly had no attacks for six months, with 60% having no attacks for twelve months.
Altogether, lanadelumab was shown to be extremely effective in managing hereditary angioedema symptoms and attacks.
Hereditary Angioedema and Immune Thrombocytopenia (Abstract 333)
Hegazi and Keith’s research seeks to understand the intersection of HAE and immune thrombocytopenia (ITP). Thrombocytopenia occurs when someone does not have enough platelets, blood cells that clot blood and can stop bleeding. Individuals with thrombocytopenia may experience prolonged bleeding, fatigue, bleeding under the skin, and jaundice. Find out more about thrombocytopenia.
While the two are usually not linked, this study was performed on a 37-year-old woman with both conditions. She was taking C1-inhibitor therapy for HAE symptoms every three months. Those with HAE often have too much C1 and not enough C4. Researchers assert that her C1 inhibitor levels, as well as her endogenous C4, were low. After two and five years, respectively, she developed low platelet counts which were diagnosed as ITP.
By increasing her C1-inhibitor therapy, the ITP issue was handled. However, researchers state that ITP can be connected to HAE, as those with too little C4 are at risk of a similar occurrence.






