A news release from Benzinga shares that biotechnology company Emerald Health Pharmaceuticals is beginning a Phase 2 clinical study on EHP-101 as a treatment for patients with systemic sclerosis.
About Systemic Sclerosis
Systemic sclerosis, sometimes called systemic scleroderma, is a rare autoimmune disorder which causes a thickening of the skin. Fibrosis, a buildup of scar tissue caused by excess collagen, occurs on the skin and organs. Other symptoms of systemic sclerosis include swelling in the hands, open sores, joint problems, diarrhea, painful lumps under the skin, and Raynaud’s phenomenon. The latter causes fingers and toes to change colors in response to temperature changes.
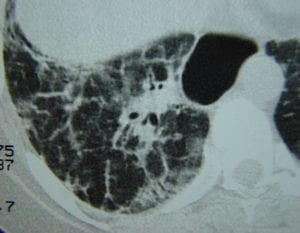
Systemic sclerosis can be considered diffuse cutaneous or limited cutaneous depending on how much it impacts the skin versus the organs. The former heavily affects both skin and organs. In severe cases, fibrosis can affect the heart, lungs, kidneys, and esophagus, leading to organ failure. Adults with ovaries are most likely to experience systematic sclerosis. Learn more about systemic sclerosis on our website.
Phase 2 Clinical Study
Emerald Health Pharmaceuticals
Emerald Health Pharmaceuticals is a biotechnology company utilizing synthetic cannabidiol and cannabigerol to target and treat autoimmune, fibrotic, and central nervous system diseases and disorders. EHP-101 is focused on treating systemic sclerosis and multiple sclerosis, while an additional drug candidate, EHP-102, is set to be tested for the treatment of Huntington’s disease and Parkinson’s disease.
The Study
The double-blind, placebo-controlled Phase 2a international clinical study seeks to understand how to effectively treat patients with systemic sclerosis. It will examine 36 patients from the United States, New Zealand, and Australia. The study follows EHP-101, an oral medication that has received Orphan Drug designation. Additionally, the study will examine EHP-101’s safety, tolerability, and treatment efficacy for patients with diffuse cutaneous systemic sclerosis.
Participants will receive four doses of EHP-101 daily, taken either all at once or twice throughout the day. This dose schedule is based on a Phase 1 study. In the Phase 2 study, researchers also look to understand patient-reported outcomes, biomarkers related to disease progression, and quality of life assessment. They will examine lung capacity and thickness as well.
Dr. Joachim Schupp notes that the rapid spread of COVID-19 does present some challenges, but Emerald Health Pharmaceuticals is committed to the health and safety of all study participants.
EHP-101
EHP-101 is a patented formulation:
a synthetic aminoquinone derivative of CBD endowed with dual peroxisome proliferator-activated receptor gamma (PPARɣ) and cannabinoid receptor type 2 (CB2) agonist activity.
PPARɣ and CB2 are both therapeutic targets, or biological targets, for systemic sclerosis. Basically, EHP-101 uses its formulation to modify cellular and immune activity causing fibrosis and other symptoms. It also targets the hypoxia inducible factor (HIF) pathway, which plays a role in how the body reacts to low oxygen environments or situations.






