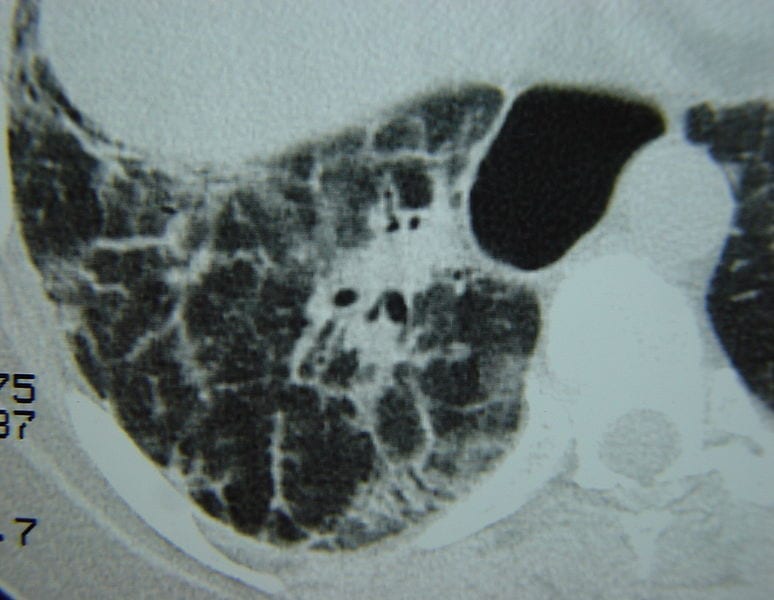
This Drug Could Prevent PAH in People with Scleroderma
According to a story from Scleroderma News, findings from the recent Phase 2 EDITA clinical trial suggested that ambrisentan (marketed as Letairis), which is approved to treat pulmonary arterial hypertension…