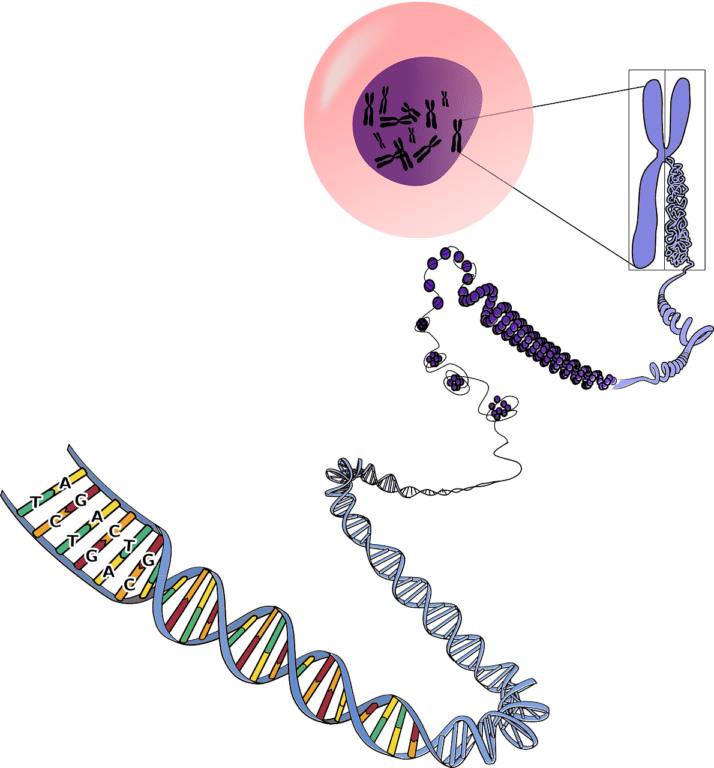
A Gene Therapy Trial for Fabry Disease Has Begun Dosing Patients
According to a story from Fabry Disease News, the first patient in a clinical trial testing the investigational gene therapy FLT190 has reportedly been dosed. FLT190 is being developed as…