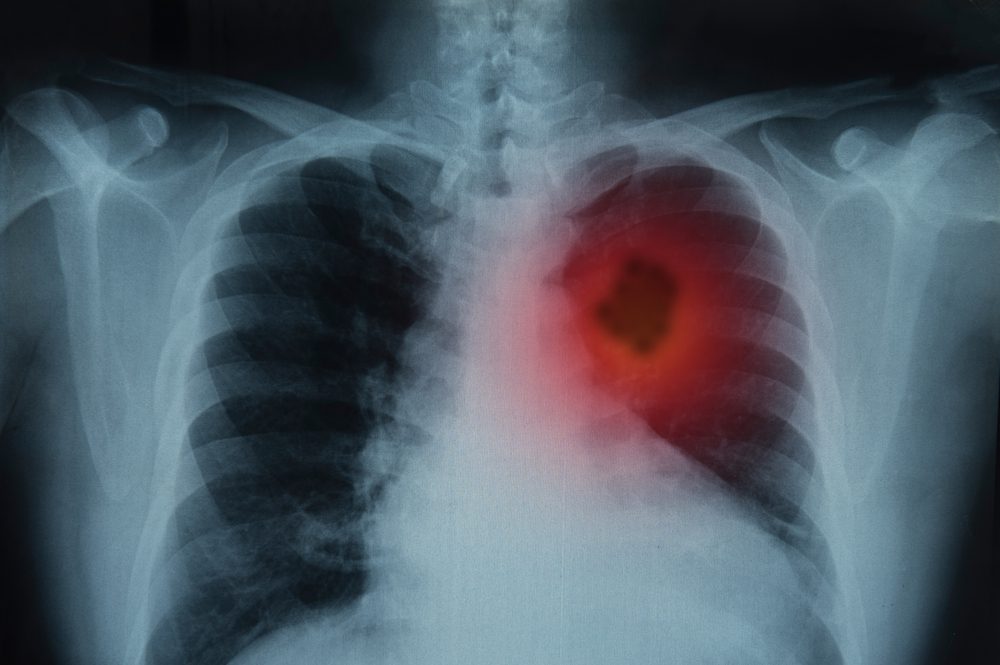
June 2025 FDA Oncology Approvals: Expanding Options and Personalization for Cancer Patients
June 2025 marked a month of significant progress in cancer care, as the FDA approved several new therapies and treatment combinations across a range of cancer types. As reported by…