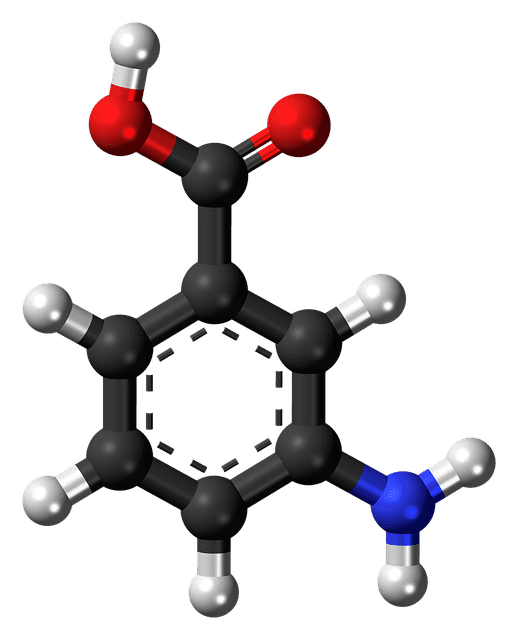
First Patient Dosed: BBP-671 for Propionic Acidemia
According to a news release from biopharmaceutical company BridgeBio Pharma, Inc., the first patient was dosed in a Phase 1 study. Researchers are evaluating the safety, tolerability, pharmacodynamics, and pharmacokinetics…