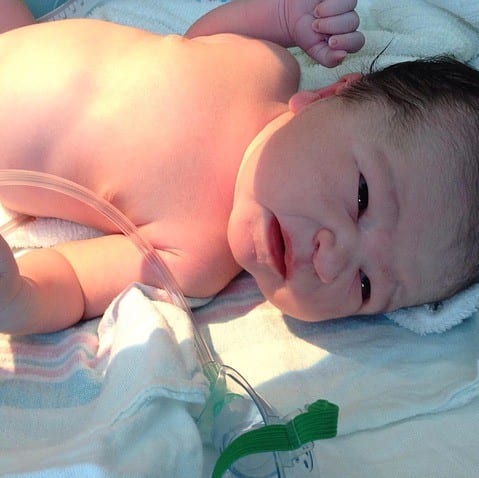
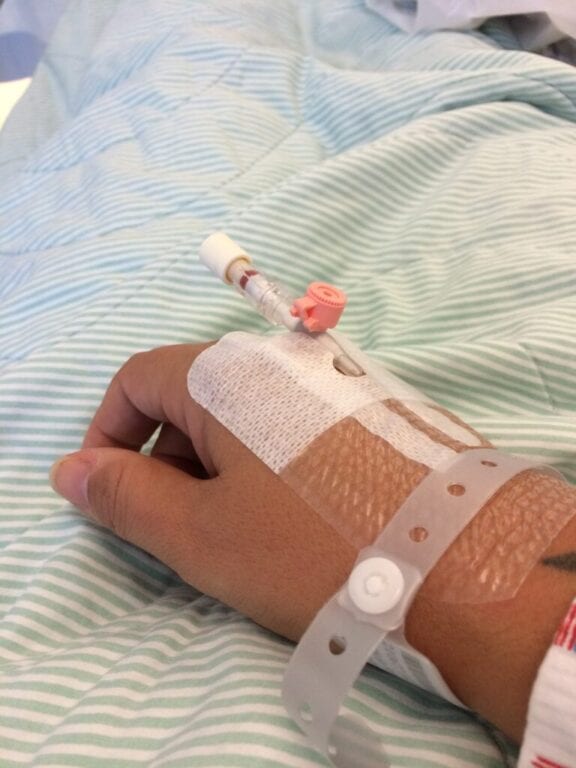
Unseen and Underdiagnosed: Betrice’s Fight to Raise Awareness of Alpha-1 in the Black Community
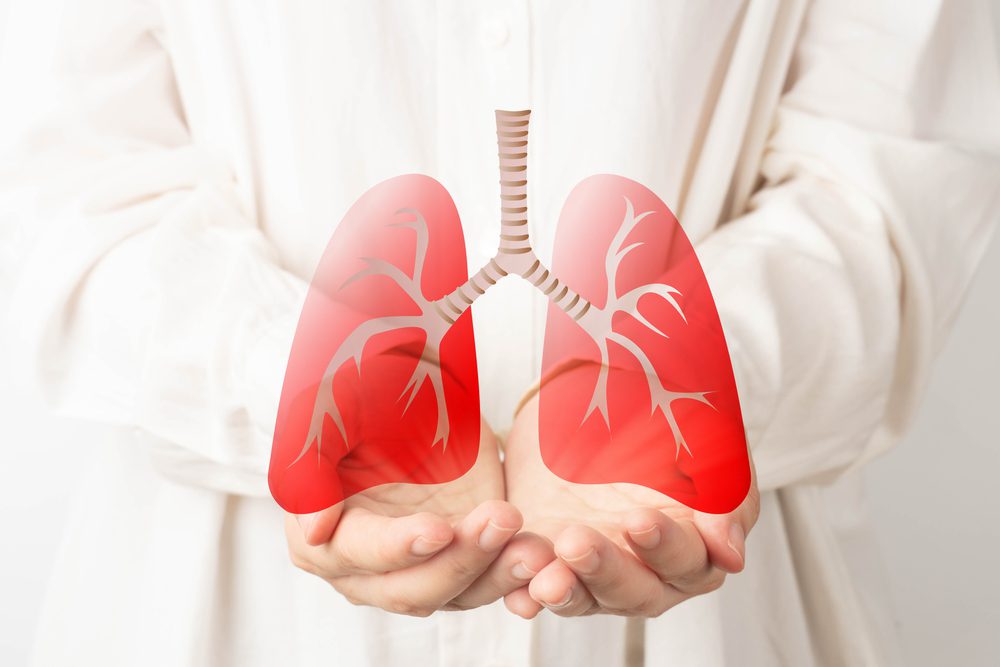
Alpha-1 Antitrypsin Deficiency: According to the Alpha-1 Foundation, 1 in 1,500 to 3,500 people of European descent are affected by this rare genetic disease. Antitrypsin or A1AT is a plasma…







.png)