Emedgene Starts Initiative to Help Undiagnosed Rare Disease Patients
According to a story from Biospace, in Palo Alto, AI company Emedgene plans to dive into helping the undiagnosed with their new rare disease initiative. The company will be paired…
According to a story from Biospace, in Palo Alto, AI company Emedgene plans to dive into helping the undiagnosed with their new rare disease initiative. The company will be paired…

COVID-19 and its rapid global spread have been anxiety-inducing, to say the least. For patients with rare diseases and their families, the growth of COVID-19 has also prompted additional…
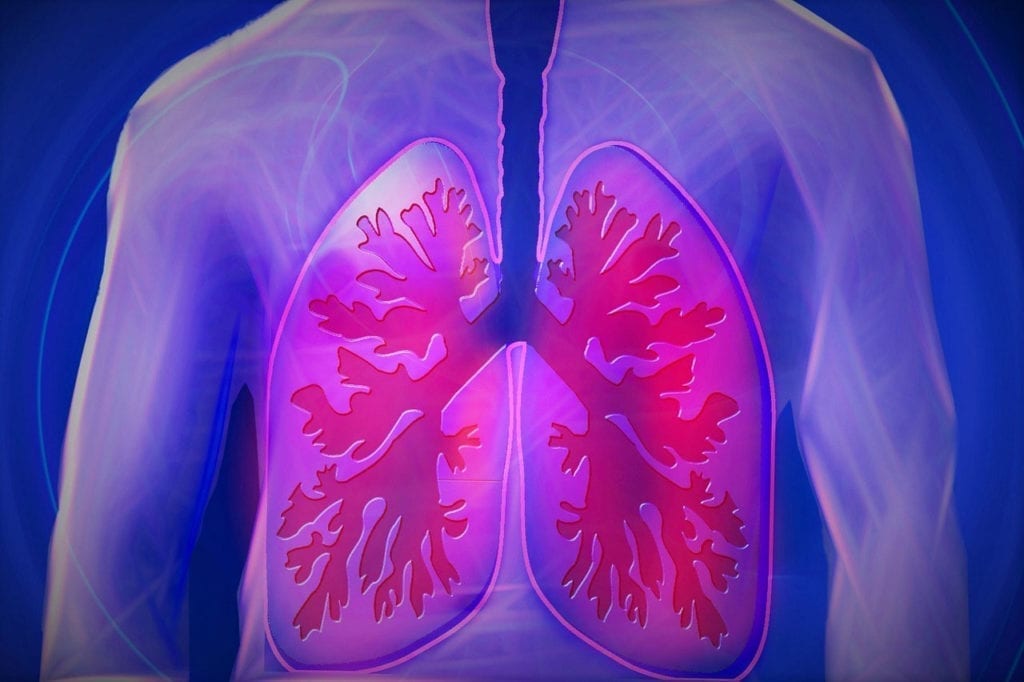
According to a release on Biospace, the European Commission (EC) has approved Takeda Pharmaceutical's ALUNBRIG (brigatinib) as treatment for patients with anaplastic lymphoma kinase-positive (ALK+) advanced non-small cell lung cancer…
If you have read the news at all lately, you know that ventilators are a sought-after piece of medical equipment. With hospitals looking to treat mass amounts of patients…
Rare, neurologic diseases often do not have viable treatments. Gaucher disease and Parkinson's disease are two examples. In situations like these, medical professionals look to treatments for other conditions, such…
Shannon and Todd Palmatier have three children, two of which have progressive familial intrahepatic cholestasis (PFIC). Their sons' diagnoses have brought the lack of knowledge and advocacy to the attention…
Social Distancing Social distancing has been coined as the most surefire way to stop the spread of COVID-19 across the American populace. What is this phrase used to refer to?…
No one was given a prep course on how to handle quarantine, especially not with kids. Here are some suggestions to help you get through these unique times, while making…
As Patient Worthy readers, we hope you and your families are doing well during this challenging time. The health and safety of our rare community is always at the…