Making a Major Impact on B Cell Cancers
Accutar Biotechnology, Cranbury, New Jersey recently announced via BioSpace that dosing has begun with the first patient enrolled in the Phase 1 trial of AC0676 a chimeric degrader molecule…
Accutar Biotechnology, Cranbury, New Jersey recently announced via BioSpace that dosing has begun with the first patient enrolled in the Phase 1 trial of AC0676 a chimeric degrader molecule…
Having a rare or uncommon condition can be undoubtedly overwhelming. The diagnosis comes with associated mental health, financial, physical, and social burdens that can weigh heavy on your mind. But…
According to a story from Market Screener, the drug company AbbVie recently announced that its investigational therapy epcoritamab has received a positive opinion from the European Union's Committee for Medicinal…

According to a July 11 article from Maaisha Osman, commercial-stage biotechnology company ADC Therapeutics has voluntarily halted enrollment for the Phase 2 LOTIS-9 study evaluating Zynlonta (loncastuximab tesirine-lpyl) and…
In March the FDA’s expert advisers voted 11 to 2 in favor of approving Roche’s antibody drug Polivy to treat lymphoma patients. According to an article in BiopharmaDive, the decision…
In December 2022, Representative Jamie Raskin (D-MD) shared with the public that he had been diagnosed with diffuse large B-cell lymphoma (DLBCL). Although being diagnosed with cancer can be frightening,…
Previous research presents strong evidence favoring shared decision-making after a medical diagnosis. One major improvement has been designing treatment plans that include a good deal of the patient’s goals and…
Due to varied periods of decline among patients, previous research indicates that it is difficult to develop treatment options for patients who have relapsed DLBCL. This holds true especially when…
According to a story from Cancer Network, the results of a recent retrospective study have found that a two-part combination treatment for diffuse large B-cell lymphoma (DLBCL), a rare cancer,…
According to a case-based roundtable meeting for Targeted Oncology, Matthew A. Lunning, DO, talked about second-line therapies for diffuse large B-cell lymphoma and the results of a study called L-MIND.…
Mark Hoppus has been working in the music industry for three decades; his band, Blink 182, was formed back in 1992. He has a strong love for writing, performing, and…
According to a recent article from Hematology Advisor, a study shows patients diagnosed with particular subtypes of diffuse large B-cell lymphoma (DLBCL) respond better to ibrutinib with R-CHOP compared to…
According to a recent article, new evidence shows adding ibrutinib to a patient’s chemotherapy regimen may improve the longevity of younger patients with diffuse large B-cell lymphoma (DLBCL). Diffuse Large…
According to a news release from immuno-oncology company IMV Inc., the first patient has been dosed in the Phase 2b VITALIZE clinical trial. During the trial, researchers are evaluating a…
According to the Cancer Network, a CAR T-cell therapy called C-CAR039 recently earned both Fast Track and Regenerative Medicine Advanced Therapy (RMAT) designations from the U.S. Food and Drug Administration.…
According to Healio, the FDA recently granted Orphan Drug designation to nanatinostat and valganciclovir ("Nana-val") for the treatment of patients with Epstein Barr virus+ diffuse large B-cell lymphoma (DLBCL). In…
IMV Inc. recently presented two posters at the AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics showcasing their DPX delivery platform's immunotherapeutic capabilities. IMV Inc. IMV Inc. is…
Written by Robyn Stacy-Humphries Wolverine, Spiderman, Wonder Woman, and Deadpool - superheroes most of us love. Why? They have superpowers and escape death by some type of genetic alteration. Humans…
CAR T-cell therapy has been used as a treatment for a variety of rare diseases including relapsed or refractory diffuse large B-cell lymphoma (DLBCL), non-Hodgkin lymphoma (NHL), as well as…
Results from a recent Phase 3 trial for diffuse large B-cell lymphoma (DLBCL) have just been released! This trial was called POLARIX and the results demonstrated that polatuzumab vedotin-based treatment…
Gilles Salles works at the Memorial Sloan Kettering Cancer Center as the Lymphoma Service Chief. She recently sat down to discuss the necessity of new treatments for diffuse large B-Cell…
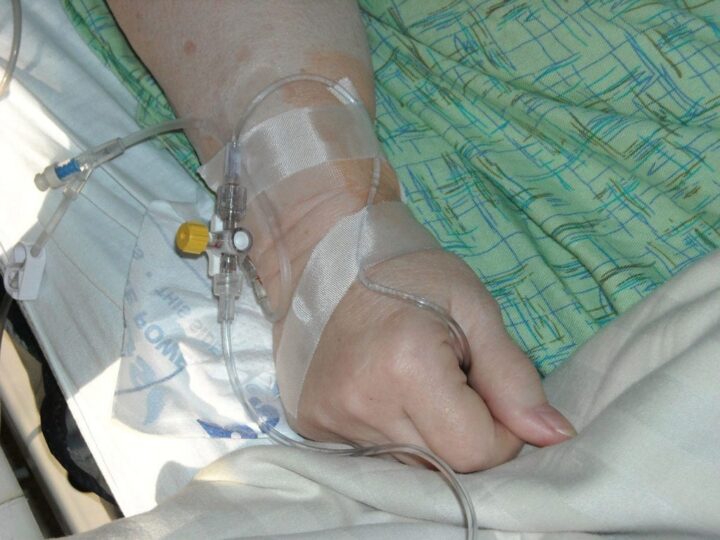
In the Phase 2 LOTIS-2 clinical trial, researchers evaluated the safety, efficacy, and tolerability of Loncastuximab tesirine for patients with aggressive diffuse large B-cell lymphoma (DLBCL). While there are treatment…
A Phase 3 clinical trial for Non-Germinal Center Diffuse Large B-Cell Lymphoma in patients where it was previously untreated have just announced they are still recruiting participants. The trial is…
According to a story from USA Today, Mark Hoppus, bass player for the iconic pop-punk band Blink-182, was recently diagnosed with a rare cancer: diffuse large B-cell lymphoma. His cancer…
Clinical trials can be crucial in determining potential treatment options for a variety of conditions. According to Pharma Biz, biopharmaceutical company Nordic Nanovector ("Nordic") recently shared promising data from the…