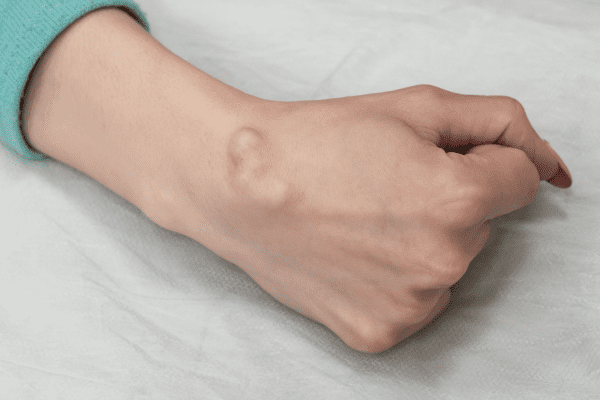
FDA Approves Koselugo for Adults with Neurofibromatosis Type 1
The U.S. Food and Drug Administration (FDA) has approved Koselugo (selumetinib), an oral MEK inhibitor developed by Alexion, AstraZeneca Rare Disease, for adults with neurofibromatosis type 1 (NF1) who have…