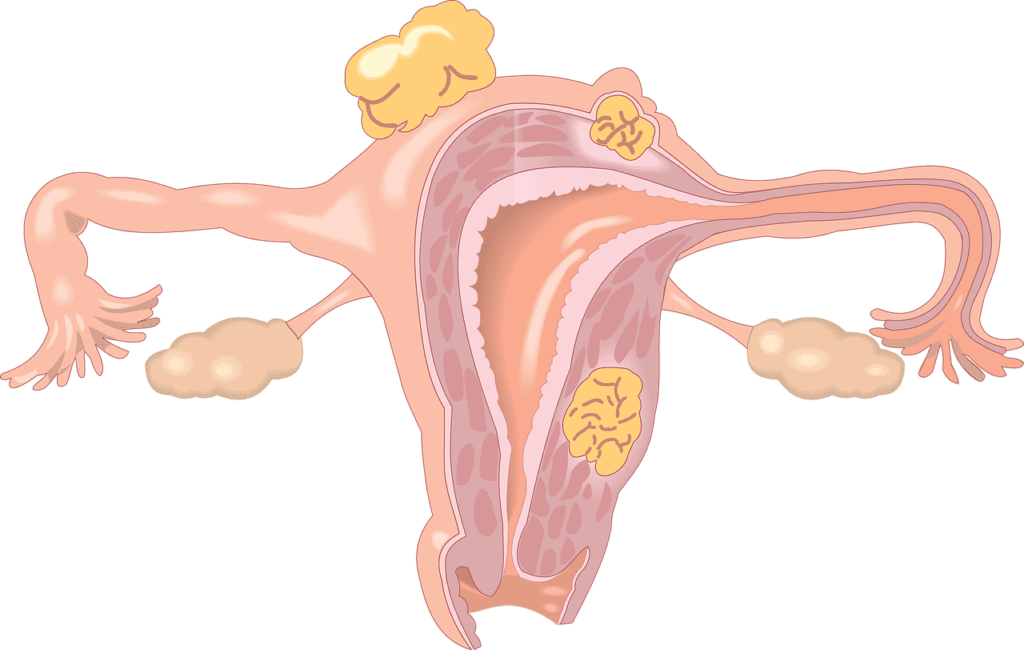
Results of Three Clinical Trials Investigating Secondary Surgery For Recurrent Ovarian Cancer
Recent clinical trials have proven that treatment for relapsed ovarian cancer is not a standard “one size fits all." After a relapse, areas where tumors spread and grow in the…