Study Shows Genetic Testing May Help Diagnose Chronic Kidney Disease
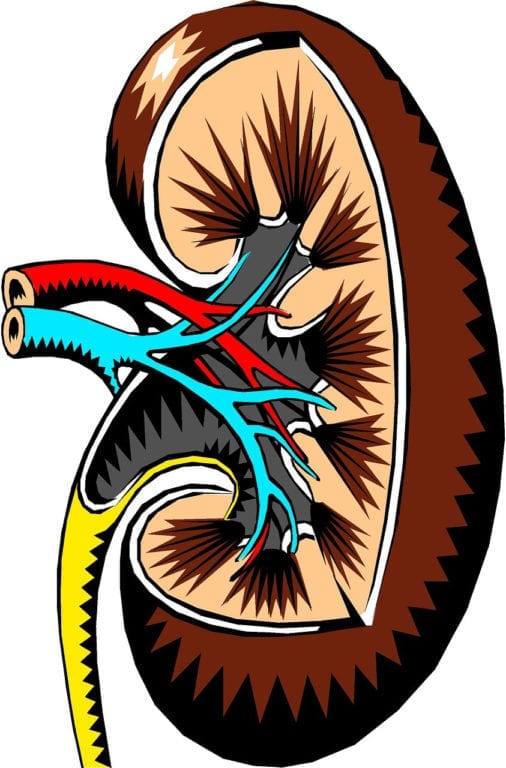
Chronic kidney disease (CKD) affects approximately 26 million people in the United States. CKD usually develops after a patient has already battled a different illness which has placed a toll on…