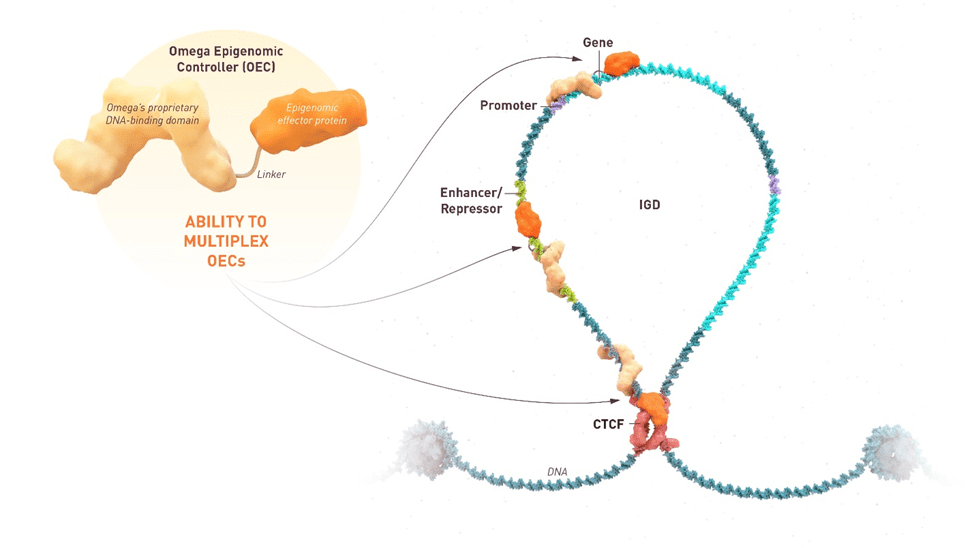
ISB 1442 for Multiple Myeloma Earns Orphan Drug Designation
Cases of multiple myeloma seem to be rising across the country and the globe. New and effective therapeutic interventions have the potential to significantly benefit this patient population. Ichnos Sciences…