Parents in Malta Handed a Prison Sentence for the Death of Daughter Who Died of Aplastic Anemia
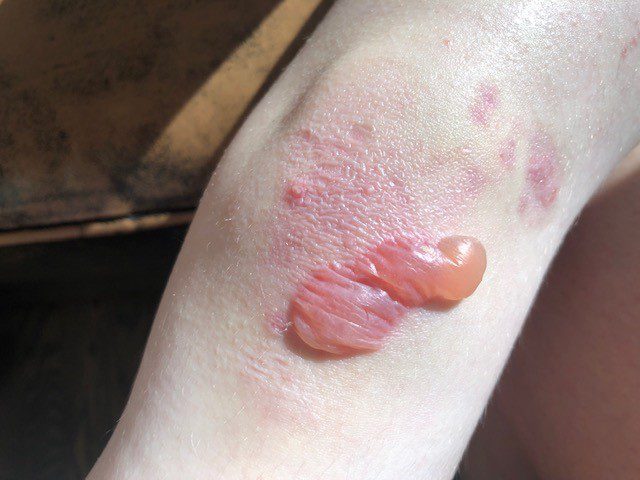
A court in Zabbar, Malta found the parents of seven-year-old Victoria guilty of her death by negligence. The court also determined that the system had failed the child by…