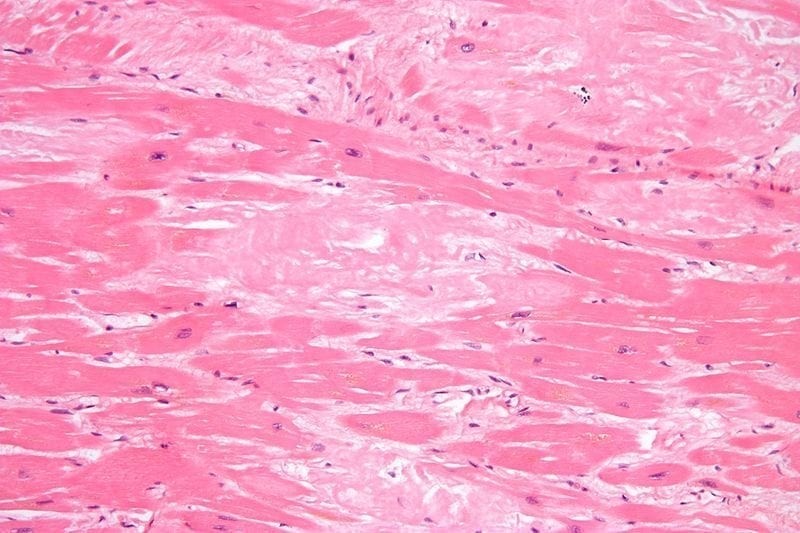
Enrollment Complete in Vutrisiran Study for ATTR Amyloidosis
On August 9, 2021, biopharmaceutical and RNAi therapeutics company Alnylam Pharmaceuticals, Inc. (“Alnylam”) shared that enrollment is now complete for the Phase 3 HELIOS-B clinical trial. During the trial, researchers…


















.jpg)
.jpg)


