Jessica Lynn has an educational background in writing and marketing. She firmly believes in the power of writing in amplifying voices, and looks forward to doing so for the rare disease community.
Currently, treatment for multiple system atrophy (MSA) is symptomatic; there are no available therapies designed to slow disease progression. This represents a huge unmet need for this patient community.…
Continue Reading
ICYMI: First Patient Dosed in ATH434 Study for MSA
Source: Pixabay.com
To Diem Nguyen, her daughter Minh is her whole world. Diem would stop at nothing to ensure her daughter gets the help that she needs. That's why, recently, Diem…
Continue Reading
Child with Aplastic Anemia Needs Stem Cell Donor
source: pixabay.com
In 2020, Anthony Di Laura and Jackie Cucullo were over-the-moon. The married couple had just learned that Jackie was pregnant with their first child. Anthony and Jackie were thrilled…
Continue Reading
Insurance Finally Approves Surgery for Father with Pseudomyxoma Peritonei
In a recent news release, clinical-stage biotechnology company Amolyt Pharma ("Amolyt") shared that positive data was available from a proof-of-concept study evaluating AZP-3601 for hypoparathyroidism. Within this study, researchers sought…
Continue Reading
Positive Data Available on AZP-3601 for Hypoparathyroidism
22-year-old Ruchita Sahukari is currently studying Literature and Sociology at the Indira Gandhi National Open University (IGNOU). Her journey to pursuing her degree began with the assistance of non-governmental…
Continue Reading
Woman with Osteogenesis Imperfecta Advocates for More Inclusive Education
Shortly after he was born, Asa Burnside was diagnosed with Niemann-Pick disease type A, a rare genetic metabolic disorder. Since then, Asa has brought so much joy to his family’s…
Continue Reading
Uncle Walks 62 Miles to Raise Niemann-Pick Funds and Awareness

https://pixabay.com/en/baby-care-child-cute-hand-face-20339/
In September 2020, Amelia Bradford turned eight months old. Her family loved their fun, wiggly, and happy baby. But then Amelia’s mother Danielle noticed something concerning: a facial bruise around…
Continue Reading
This Baby’s Black Eye Turned Out to Be Neuroblastoma
Have you ever heard of Orphan Drug designation? In the United States, this designation is granted by the U.S. Food and Drug Administration (FDA) to drugs or biologics intended…
Continue Reading
DSP-0390 for Glioma Earns Orphan Drug Designation

Photo: https://unsplash.com/photos/qOR762W7OvA
When Eleni was first born, doctors and nurses told her parents, Neil and Eloise, that their daughter would most likely not survive. That’s because Eleni had been diagnosed with…
Continue Reading
Volunteers Abseil to Raise Funds for Girl with Nonketotic Hyperglycinemia

source: pixabay.com
Have you ever thought about walking to raise funds and awareness? Well, at the Twin Cities Team Hope Walk, you can do just that! According to Kare11, this event…
Continue Reading
Twin Cities Team Hope Walk to Fundraise for Huntington’s Disease
12-year-old Rohit, who hails from Uttar Pradesh, India, has a dream to one day become a singer – and he’s ready to pursue that dream no matter what. Throughout…
Continue Reading
Rohit’s Story: Living with Osteogenesis Imperfecta
When Nicole Croxton and Pete Nappi first learned that they were going to have another daughter, the pair were thrilled. But during the course of Nicole’s pregnancy, doctors filled…
Continue Reading
Family is Fundraising for Daughter with Osteogenesis Imperfecta
source: pixabay.com
The Marburg virus, caused by Marburg marburgvirus (MARV), was first identified in 1967 after hemorrhagic fever outbreaks occurred in laboratories in Serbia and Germany. Since its initial identification, Marburg…
Continue Reading
NEWS: Marburg Virus Outbreak Occuring in Ghana

Source: Pixabay.com
A few years ago, Dr. Thomas Weimbs, a researcher at the Weimbs Lab of UC Santa Barbara, performed a study on mice models of polycystic kidney disease (PKD). During…
Continue Reading
Could a Ketogenic Diet Benefit Those with ADPKD?
source: pixabay.com
Recently, the International Society on Thrombosis and Haemostasis (ISTH) held its 2022 Congress from July 9th to 13th. During the conference, researchers and other stakeholders discussed research, care, and…
Continue Reading
Positive Data Available: FLT180a for Hemophilia B
Primary progressive aphasia (PPA) is a rare neurological disorder and form of frontotemporal dementia which affects one’s ability to communicate. In many cases, people develop PPA prior to turning…
Continue Reading
SYDBAT App Can Help with Early Primary Progressive Aphasia Diagnosis
source: pixabay.com
Earlier this year, the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) held its International Congress. During the Congress, researchers discussed the latest in research and scientific innovation within…
Continue Reading
ICYMI: Phase 3 Trial Data Available on Lumasiran for Primary Hyperoxaluria type 1
source: pixabay.com
Currently, there are few therapeutic options for people who become infected with the Nipah virus. Rather, treatment is symptomatic. However, that may soon be changing. According to the European…
Continue Reading
Phase 1 Trial Will Evaluate mRNA-1215 Vaccine for Nipah Virus
Michael Klim was traveling three years ago when he felt like something within his body was wrong. The Polish-born swimmer, who has won two gold medals for Australia while participating…
Continue Reading
Olympic Swimmer Michael Klim Shares CIDP Diagnosis
Early diagnosis and treatment is crucial for patients with primary hyperoxaluria type 1 (PH1). Unfortunately, due to a high prevalence of misdiagnosis, as well as poor therapeutic access in…
Continue Reading
Positive Data Available on BBP-711 for Primary Hyperoxaluria type 1

source: pixabay.com
One thing is sure: 29-year-old Aimee Read was always a fighter. At just two years old, Aimee was diagnosed with leukemia. After two years of chemotherapy and six months…
Continue Reading
Woman with PNH Dies Just Weeks Before Bone Marrow Transplant
[Source: pixabay.com]
Currently, ZYNLONTA (loncastuximab tesirine-lpyl) is approved for the treatment of individuals with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) for which two prior therapies had not worked.…
Continue Reading
Dosing Begins in ZYNLONTA Trial for Non-Hodgkin’s Lymphoma
In the United States, during the drug development and approval process, drug developers are able to request Fast Track designation. This process is designed to facilitate and expedite the…
Continue Reading
NUZYRA for NTM Lung Disease from MAC Earns Fast Track Designation
source: pixabay.com
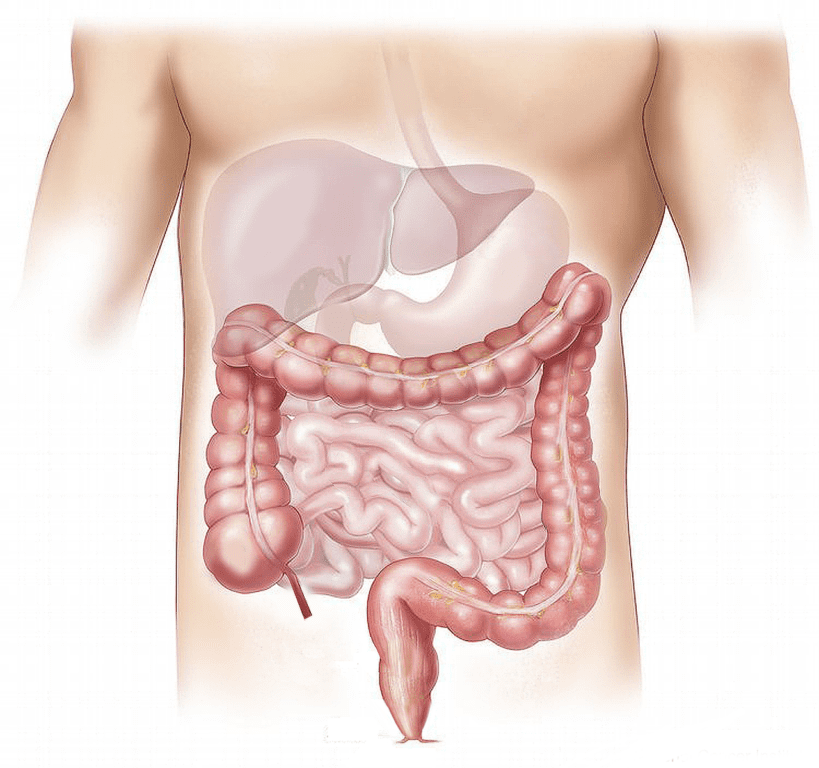
Medical research and clinical trials are extremely important tools in learning more about specific diseases, as well as understanding and evaluating potential treatments. For example, the Phase 2 VIBRANT…
Continue Reading
Preliminary Data Available on Vurolenatide for Short Bowel Syndrome

source: pixabay.com
Those living with rare diseases or conditions often face difficulty in finding treatment options - or even spurring researchers to make headway within their disease states. To combat this and…
Continue Reading
Paxalisib Earns Orphan Drug Designation for Atypical Teratoid Rhabdoid Tumors