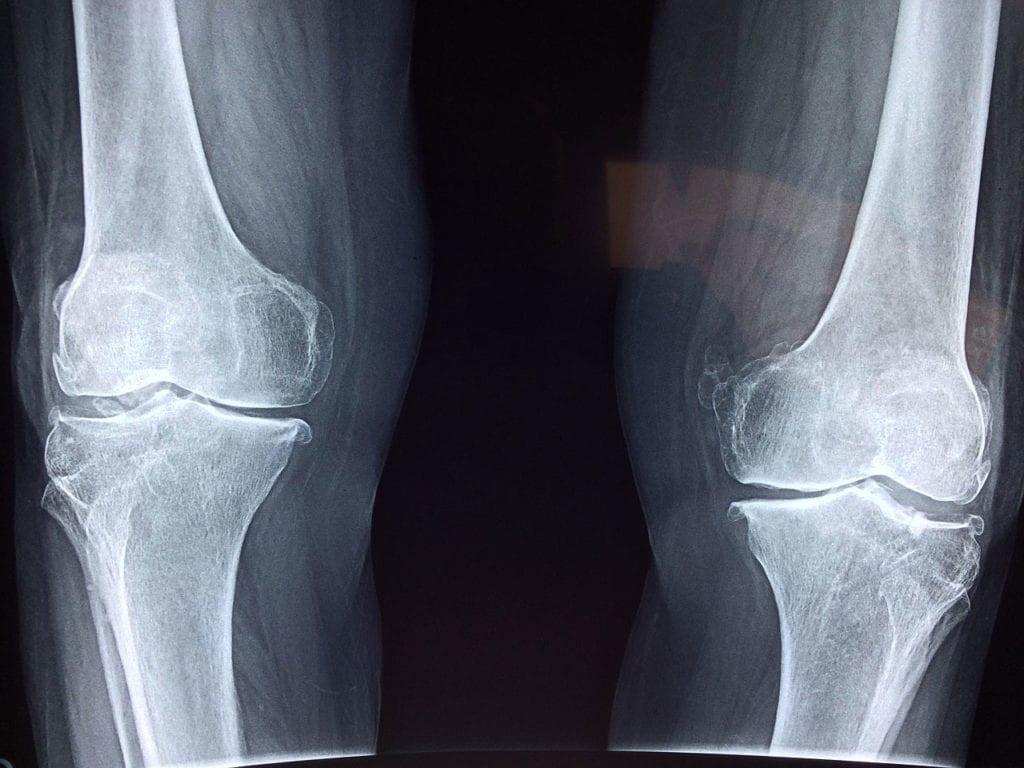
In Her 9th Osteosarcoma Relapse, This Young Woman Continues to Fight
When Camille Wahl was a child, she loved Irish step dance. She translated her love physically, becoming a competitive dancer until she was ten years old. It was then, reports…

When Camille Wahl was a child, she loved Irish step dance. She translated her love physically, becoming a competitive dancer until she was ten years old. It was then, reports…
Welcome to Study of the Week from Patient Worthy. In this segment, we select a study we posted about from the previous week that we think is of particular interest…
The first thing that you should know: osteosarcoma (a type of bone cancer) is treatable. In fact, when you treat osteosarcoma early enough, it is curable; children can live long,…
Walt McGrory always knew that he wanted to play basketball. So when he became a student at the University of Wisconsin-Madison (UW-Madison), he joined the Badgers as a walk-on. In…

Ever since the first moment he can remember, eight-year-old Dominic Gamez was drawn to travel soccer. He felt strong and powerful as his legs carried him across the field;…

The Rogers, Minnesota hockey community is strong and unified; if something happens to someone in their ranks, they step up to assist. But nothing could have prepared them for the…
Happy Monday! This week, we have stories on a potential treatment approach for an aggressive form of mitochondrial disease, an experimental treatment for osteosarcoma earning Rare Pediatric Disease designation, and…
In late September 2022, biotech company Oncoheroes Biosciences, Inc. ("Oncoheroes") shared via news release that its product dovitinib, which the company has an exclusive pediatric licensing agreement for from…

Osteosarcoma is genomically complex, which means that – sometimes – it can also be difficult to treat. Treatment and management, however, are crucial to ensuring positive patient outcomes. Doctors and…

Historically, both osteosarcoma and neuroblastoma have been difficult to treat. Therefore, new treatment options are needed for these cancers, which predominantly affect children and teenagers. According to Medical XPress, a…
The Myrovlytis Trust, a UK Charity focused on promoting research and advancing education for rare diseases, and Patient Worthy are now partners! This organization has focused primarily on Birt-Hogg-Dubé syndrome…

According to a November 3, 2021 news release from biopharmaceutical company OS Therapies, the company’s therapeutic candidate OST-HER2 (OST31-164 / Listeria monocytogenes) received Rare Pediatric Disease Designation from the FDA.…
There are two types of osteosarcoma, a rare bone cancer: localized or metastasized. If the cancer is localized, or in one area, the 5-year survival rate sits around 70-75%. However,…
What do you call fossils that just lie there? Lazy bones! But for the Centrosaurus apertus, its bones are talking to us from beyond the grave, making it less lazy and…
Cabozantinib has recently been studied as a treatment for Ewing sarcoma and osteosarcoma. It demonstrated antitumor activity in both of these cancers, which was discovered in the second phase of…
The biopharmaceutical company Cellectar Biosciences recently announced that the FDA granted its lead phospholipid drug conjugate, CLR 131, Orphan Drug Designation(ODD) for treatment of lymphoplasmacytic lymphoma. ODD is granted by…

According to a story from Duke Cancer Institute, Chantal Rose-Denman, age 44, has been a runner ever since she was a teenager. 17 years ago she made the switch to…

According to a story from Science Daily, a recent study has found that osteosarcoma, a form of bone cancer, displays similar genetic characteristics when it appears in both humans and…

According to a story from The ASCO Post, recent studies suggest that around 70,000 adolescents and young adults are diagnosed with some type of cancer in the US each year.…
According to a story from jsonline.com, Tyler Trent, a Purdue University student who captured the hearts of thousands with his struggle against osteosarcoma, passed away at age 20 after his…
Olivia Egge is an osteosarcoma cancer survivor and an advocate for all those living with rare conditions like her own. The senior in high school living in Arlington, Virginia recently…
According to a story from marketscreener.com, the drug development startup Endocyte has recently reached $1.5 billion in total value. This biopharmaceutical company has been focused on developing therapies for two…
Ailani Banuelos is a 12-year-old with a dream. She not only wants to see the ocean but paddle in it. Living in Chicago made the dream fairly difficult. There simply…

Filipa Vance, a woman who was diagnosed with osteosarcoma when she was 15, now celebrates 30 years of living post-diagnosis. Filipa is now cancer-free, but lives with many of the…

Researchers from the University of Illinois have been studying the effects of endocannabinoids (a type of molecule that occurs naturally in the body) on cancer. So far, the researchers have…