Idiopathic Pulmonary Fibrosis: Antifibrotic Therapy Can Curb Exacerbations and Mortality
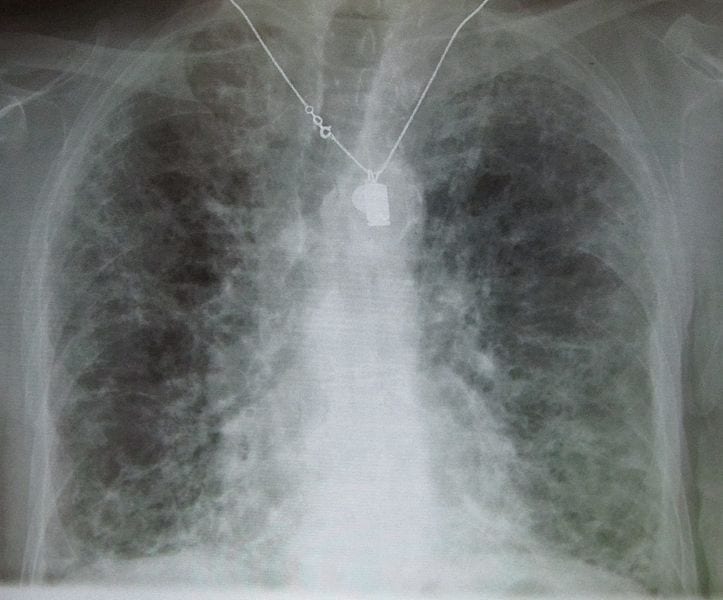
According to a story on medicaldialogues.in, a recent study has determined that antifibrotic treatment can reduce the rate of mortality and acute exacerbations in people living with idiopathic pulmonary fibrosis…