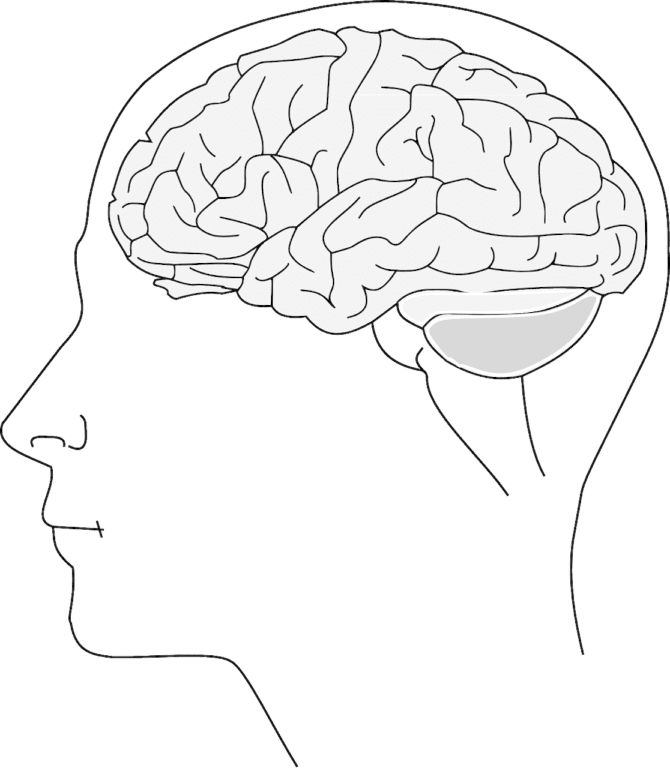
Study Shows New Possible Treatment for ALS
Copyscape score: 6% Currently there are only two FDA approved treatments for the treatment of amyotrophic lateral sclerosis (ALS). These treatments are only modestly effective, partly because ALS is not…
Copyscape score: 6% Currently there are only two FDA approved treatments for the treatment of amyotrophic lateral sclerosis (ALS). These treatments are only modestly effective, partly because ALS is not…
A new treatment has been found to improve the life of those with Parkinson's Disease tremors through a minimally invasive procedure that sends pulses of focused ultrasounds to the…
For millions of Americans who are affected by rare diseases, hope and health lies in the availability of plasma donations. One use of the donated plasma involves the extraction of…

As originally reported in Medical Xpress, CRISPR has been a hot conversation starter in recent years. It has been framed as gene editing made easy: cheaper and simpler than previous…
According to a story from BioPortfolio, Acceleron Pharma and the Bristol-Myers Squibb Company have announced recently that the US Food and Drug Administration (FDA) will review the supplemental Biologics License…
According to a story from Business Wire, the biopharmaceutical company Bristol-Myers Squibb has recently announced that the US Food and Drug Administration (FDA) has granted the company's drug abatacept (marketed…
The iPain Summit 2019 took place from November 14th-16th, 2019. Patient Worthy had the privilege to attend this event and cover it live on social media. This summit was focused…

A new clinical trial will be conducted at The Center for Neurology of the Academic Specialist Center (ASC) in tandem with the Karolinska Institute. This study is meant to…
Surufatinib was recently granted Orphan Drug designation by the FDA. This drug was made for the treatment of pancreatic neuroendocrine tumors (PanNETs). It was created by Hutchison China MediTech…
At the International Pain Foundation's iPain Summit, held this year from November 14th-16th, a wide variety of topics related to the challenges faced by patients with chronic pain were discussed.…
As reported in the Guardian, at Bath University, scientists have created a groundbreaking technology for the future of medication: artificial nerve cells or neurons created on silicon to replace deteriorating…
According to a story from tmcnet.com, the biopharmaceutical company Immunovant has just announced that beginning of patient dosing in its phase 2b clinical trial. This clinical trial will test the…
In a meeting with some of the world's brightest medical minds, the importance of personalized treatment for inflammatory bowel disease was discussed. At the 14th Annual Congress of the…
According to a story from Healio findings from three studies indicate that the drugs bimekizumab and certolizumab pegol had the potential to be useful treatments for patients with axial spondyloathritis.…
According to a story from Biotech 365, the biopharmaceutical company Avadel Pharmaceuticals plc recently announced that it has completed enrollment for its phase 3 clinical trial. This trial will test…

Copyscape score: 2% Brigham and Women's is launching the nation's first ever center to conduct research on primary sclerosing cholangitis, a rare liver disease that affects about 30,000 people in…
Happy Thursday, Readers! This week we're highlighting four articles on rare disease and chronic illness patients and ways to provide support that goes beyond treating physical symptoms. First, we have…
Three medications have been found that safely treat refractory status epilepticus, which is a disorder in which seizures continue even after being treated with common medication. Benzodiazepene medications, medications…
According to a story from PR Newswire, the biotechnology company Regeneron Pharmaceuticals, Inc., recently announced the release of data from a clinical trial comparing four experimental treatments for Ebola virus…
In a story for Drug Development & Delivery, the CEO of the biotechnology company ProQR, Daniel de Boer, recently sat down for an interview. In this interview, Mr. de Boer…
According to a story from BioPortfolio, the biopharmaceutical company Incyte Corporation has recently announced that the company's New Drug Application (NDA) was recently accepted by the US Food and Drug…

A recent meta-analyses of clinical studies and case reports that examined the safety of idursulfase therapy, which is an enzyme therapy, has shown that this form of treatment is…
Contact: Britta Vander Linden [email protected] 917.604.6518 30 million Americans need more treatment and diagnostic opportunities https://everylifefoundation.org/rare-disease-community-calls-on-congress-fda-to-enact-life-saving-public-policy-solutions/ (Washington, D.C., December 4, 2019) Hundreds of rare disease advocates from around the country were brought to Washington,…
Getting a diagnosis for a rare disease is hard. Not many people are familiar with the many obscure symptoms and manifestations of these diseases. Because of this, patients with rare…
A recent article in the Taunton Gazette describes the dilemma faced by the parents of four-year-old Jaxtien Miller who is a metachromatic leukodystrophy patient waiting for a stem cell…