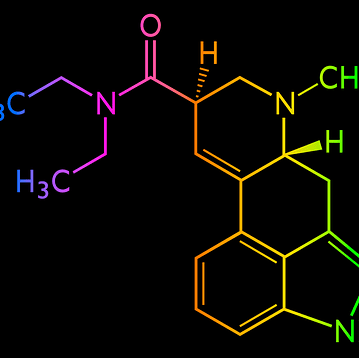
Animal Study Will Evaluate SLS-007 as a Treatment for Parkinson’s Disease
Researchers at Seelos Therapeutics are planning an animal study to evaluate SLS-007, a treatment for Parkinson's disease. According to an article from Parkinson's News Today, this study is possible after…